CHAPTER 16 Miscellaneous Viral Respiratory Diseases
EQUINE ADENOVIRUSES
Etiology
Equine adenoviruses are members of the genus Mastadenovirus, family Adenoviridae. Only a single antigenic type of equine adenovirus (EAdV1) has been isolated from horses with respiratory disease.1,2 A second serotype, equine adenovirus type 2 (EAdV2), has been isolated from the feces of foals with diarrhea.3 The biophysical properties of EAdV are similar to those of adenoviruses of other species. Equine adenoviruses are nonenveloped, 70 to 80 nm in diameter, and the capsid is composed of 252 capsomers; 240 hexamers occupy the faces and edges of the 20 equilateral triangular facets of an icosahedron, and 12 pentamers occupy the corners. The inner core contains the double-stranded deoxyribonucleic acid (DNA) genome, which for EAdV1 is 34.4 kilobases in length. Restriction endonuclease maps and genome orientation data were published for EAdV1,4 and genomic sequence data for both viruses were also published.5,6 Nucleotide sequence data for the EAdV2 genome corroborated at the molecular level that EAdV2 is distinct from EAdV1 and that the two viruses evolved separately.5
An adenovirus-associated virus was isolated from a foal with respiratory disease after inoculation of equine cell cultures.7 As for adenovirus-associated viruses of other species, the equine virus is assumed to be nonpathogenic.
Epidemiology
EAdV1 occurs worldwide, and seroprevalence rates vary from less than 2% to 100%, depending on the serologic test used and the age, breed, activity, and size of the population sampled reported.2 The prevalence of antibody to EAdV1 increases with age such that in some populations, about 70% of yearlings and 2-year-old horses have EAdV1 antibody. Serologic evidence indicates a high infection rate in the first year of life, but in some populations, 50% of horses under 1 year lack EAdV antibody and are presumably at risk for EAdV disease.1
All adenovirus infections appear to be followed by a latent carrier status and shedding of the virus. Studies in the Pirbright, United Kingdom, pony herd indicated that EAdV1 infections were often subclinical, and that virus may persist in the nasopharyngeal mucus for up to 68 days after primary infection.8 EAdV1 was isolated from a foal, without clinical signs at the time of isolation, at 3 days of age.9 It seems that foals may acquire infection from their dams or other horses in their cohort during the suckling period, even in the presence of detectable levels of maternal antibody. Thus, if there is persistent or repeated EAdV infection, the passive immunity of the foal would be converted to an active immunity, probably without significant clinical disease. Disease would develop if primary infection occurred after maternal antibody had declined, or if the foal was unable to produce an active immune response. This overview of the early natural history of EAdV1 is supported by the natural history of EAdV1 in Arabian foals with PSCID, in which the transmission pattern and consequences of infection are viewed in the absence of an active immune response.10
EAdV2 has been isolated in Australia and New Zealand.3,11 Collected from widely separated geographic areas in Australia and from horses of diverse breed and age, 327 horse serums were tested for EAdV2 neutralizing antibody; 77% of these serums were positive (maximum titer, 1:640). This pattern of infection is likely to occur worldwide.
Pathogenesis
EAdV1 presumably is frequently acquired as a droplet or close-contact respiratory or ocular infection. The virus replicates in epithelial cells throughout the respiratory tract, producing lysis and sloughing of these cells and a hyperplastic response in underlying noninfected cells. Respiratory disease in foals is more severe and more likely to be associated with pneumonitis when there is total or partial failure of maternal antibody transfer (T.B. Crawford, personal communication). Recovery from disease caused by EAdV1 alone in immunologically competent horses occurs within a week to 10 days, but mixed infections with other viruses and bacteria may cause more severe and prolonged disease.8,12 Multiple infections involving various combinations of equine herpesviruses types 1 and 4, equine rhinitis A and equine rhinitis B viruses, and equine adenoviruses were recognized in 15 of 69 outbreaks of respiratory disease of horses in the United Kingdom.13 EAdV1 may infect cells of the GI tract and may be shed in feces, and presumably it may also be transmitted through a fecal-oral cycle. EAdV1 was isolated from neuritis of the cauda equina but was probably an incidental contaminant.14
Immunity
Immunocompetent foals develop EAdV1 antibody, recover spontaneously from the disease, and generally do not possess recoverable virus by day 10 after infection.12 In experimental infections of colostrum-deprived and colostrum-fed foals, the colostrum-fed foals had less severe changes than colostrum-deprived foals.12,17
Reinfection by EAdV1 was found to occur frequently in a group of 16 mares and foals, and many of these infections (or reinfections) occurred in the presence of high levels of circulating antibody.22 Immunoglobulin A (IgA) in nasal secretions is responsible for resistance to reinfection in human infections with adenovirus or rhinoviruses. A similar situation could occur in horses, when rapidly declining levels of nasal antibody after an infection could soon render the horse susceptible to reinfection, despite high serum antibody levels.
After intramuscular (IM) immunization with live EAdV1 and subsequent development of high serum antibody levels, a specific-pathogen-free (SPF) foal proved resistant to intranasal challenge with EAdV1.17 Clinical disease did not develop and virus was not isolated, although there was a greater than twofold increase in serum-neutralizing (SN) antibody after challenge. Immunity was correlated with prior exposure to the virus and high circulating SN antibody levels.
An experimental inactivated EAdV1 vaccine elicited high antibody titers in rabbits, mice, and foals. Using nude mice as a model of T-cell immunodeficiency, it was shown that production of EAdV1 SN antibody and, to a lesser extent, hemagglutination-inhibiting (HI) antibody was T lymphocyte dependent.23 As a measure of cell-mediated immune responses, an EAdV1-specific in vitro lymphocyte blastogenesis assay was developed and evaluated using lymphocytes from four vaccinated and two unvaccinated control horses. The four vaccinated horses showed marked increases in stimulation indices in response to EAdV1 antigen (maximum stimulation indices, 5.3-18.6).24
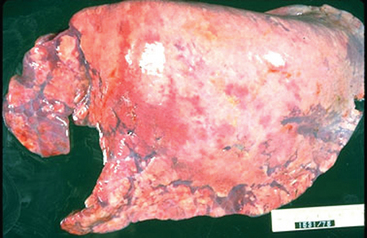
As mentioned, EAdV1 is peculiarly associated as a dominant pathogen in the uniformly fatal, inherited disease syndrome, PSCID.25,26 When first recognized in the early 1970s, PSCID was estimated to cause the death of about 3% of all purebred Arabian foals. Foals are born with a total absence of T and B lymphocytes, and PSCID is inherited as an autosomal recessive gene.27,28 A consistent and dominant feature of PSCID is an inexorably progressive EAdV1 bronchopneumonia (Fig. 16-1); the virus also causes pathology in a wide variety of other organs and tissues in PSCID foals, including the GI tract, liver, pancreas, and bladder.
Clinical Findings
EAdV1 causes acute upper respiratory disease with nasal discharge, conjunctivitis, and bronchopneumonia and infection of the GI tract, leading to the production of soft feces. Other clinical signs include coughing after exercise and enlarged submandibular lymph nodes.2,13 Severe or fatal bronchopneumonia has been occasionally recorded in non-immunodeficient Thoroughbred foals.15,16
Respiratory disease signs were described in an experimentally infected SPF foal that was cesarean delivered, colostrum deprived, and artificially reared in an EAdV-free environment.17 The foal was healthy and 6 weeks old when infected and had been shown to be immunocompetent. After intranasal infection with EAdV1, clinical signs included mucopurulent nasal discharge, severe follicular conjunctivitis (Fig. 16-2), transient anorexia and pyrexia, and sustained tachypnea. There were no changes in blood leukocyte numbers. EAdV1 was readily isolated from nasal, conjunctival, and rectal swabs and from lung, trachea, bronchial lymph nodes, and small intestine tissue homogenates obtained after an elective postmortem at 6 days after infection.
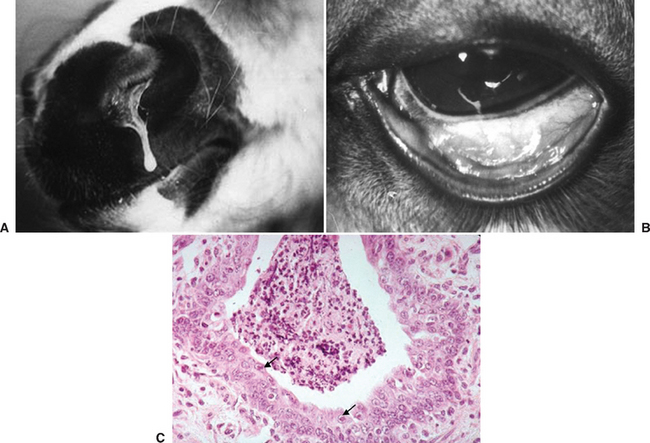
Fig. 16-2 A, Nasal discharge in 9-week-old specific-pathogen-free (SPF) foal 6 days after experimental intranasal/intraocular infection with EAdV1.17 B, Conjunctivitis in the same foal 6 days after infection. C, Low-power image of bronchiolitis of the same foal. Note proliferation and disorientation of bronchial epithelial cells and the highly cellular bronchial exudate. Arrows indicate adenovirus inclusion bodies in nonsloughed bronchial epithelial cells.
The clinical response to experimental infection of a 4-day-old foal that received colostrum but was artificially reared from 12 hours after birth in an EAdV1-free environment, and that had a 1:320 SN antibody titer and a 1:40 HI antibody titer, was similar to that of the previous foal, except fever was not as marked or as sustained, the conjunctivitis was less severe, and nasal discharge was minimal.17 EAdV1 was readily isolated from nasal and conjunctival swabs and from trachea and lung, but not from rectal swabs, small intestine, or bronchial lymph nodes, when an elective postmortem was conducted 6 days after infection.
The production of soft feces in adult horses, indicative of GI infection, has been reported as a sole manifestation of EAdV infection.13 Replication of EAdV1 in cells of the GI tract was confirmed after experimental intranasal infection.17
EAdV1 was reported as a potential cause of abortion in mares, and abortion was reproduced experimentally after intrauterine inoculation of the virus.12 However, claims for the natural occurrence of EAdV abortion are unsubstantiated.
EAdV2 was isolated from foals with severe diarrhea in which rotavirus was also present.18 Several foals died, and at postmortem one had an intussusception. In light of experiences with a human rotavirus vaccine that was withdrawn from the market because of an increased incidence of intussusception in infants receiving the live-virus oral vaccine, it seems of interest that the 1976 case of intussusception in the foal, to our knowledge, was the first in any species associated with adenovirus/rotavirus infection.
Diagnosis
Clinical Laboratory
Virus isolation from nasopharyngeal and conjunctival swabs during the acute phase of infection is possible but not frequently reported. EAdV1 may also be isolated from rectal swabs but would need to be differentiated from EAdV2. Polymerase chain reaction (PCR) primers have been designed for the detection of both EAdV1 and EAdV2.19 Detection of adenovirus in negatively stained preparations from fecal samples by electron microscopy (EM) is readily achieved. Immune precipitation, complement fixation, hemagglutination, HI, and SN assays have been extensively used in diagnosis and seroepidemiologic studies. EAdV1 hemagglutinates human blood group O and equine erythrocytes, but not those of sheep or chicken.20 EAdV2 does not hemagglutinate human O, rhesus macaque, or equine erythrocytes, and accordingly, HI assays for EAdV2 have not been developed.3
Virus Isolation
EAdV1 and EAdV2 are highly host cell specific and have been cultivated only in cells of equine origin in which both viruses produce a cytopathic effect. On light microscopy and hematoxylin-eosin (HE) staining, intranuclear inclusion bodies are a prominent feature of the cytopathology of adenovirus-infected cells. On thin-section EM, virions assembled in the nucleus form crystalline aggregates.
Serology
Adenoviruses are typed on the basis of SN assays. Most adenoviruses hemagglutinate appropriately chosen red blood cells (RBCs), and HI assays are used for antibody detection. Hemagglutination is mediated by the knoblike tip of the penton binding to receptors on the RBC surface. Type-specific antigenic determinants defined by SN and HI assays are located on the outward-facing surface of the hexamers. EAdV1 possesses the common group-specific mastadenovirus antigen.20 HI antibody to EAdV1, by definition, is type specific.3,20 Extensive analysis of adenoviruses recovered from horses with respiratory disease, including PSCID Arabian foals, indicated that on SN and HI assays, all were a single antigenic type, designated EAdV1.1,21 On SN assay, EAdV2 is unrelated to EAdV1.3
Pathologic Findings
As previously described, after experimental infection the colostrum-deprived SPF foal showed gross and histopathologic evidence of rhinitis, conjunctivitis, tracheitis, and pneumonia. There was both bronchopneumonia and interstitial pneumonia in affected areas of lung. Duodenal villous atrophy and idiopathic glomerular hyperplasia were also observed. EAdV antigen was detected by indirect immunofluorescence antibody staining of trachea and lung, but not in frozen sections of bronchial lymph node or small intestine. In the SPF foal that received colostrum, gross and histologic evidence of EAdV1 disease was generally similar but less severe than that observed in the SPF colostrum-deprived foal.17
HENDRA VIRUS
Hendra virus, formerly known as equine morbillivirus, was first recognized in 1994 as the cause of an outbreak of acute respiratory disease that affected 21 Thoroughbred racehorses in Hendra, a suburb of Brisbane, Queensland, Australia. Fourteen horses died, and seven in-contact horses were euthanized. The trainer of the horses and a stable hand were infected with the virus and became seriously ill, and the trainer died.29 A second occurrence of the disease, in Mackay, Queensland, involved two horses that died and a second human death.30,31 Three further incidents of single, sporadic deaths in horses, as well as a suspected infection of a veterinarian who autopsied one of the horses and who recovered, all in northern Queensland, have been recorded.32–34
Etiology
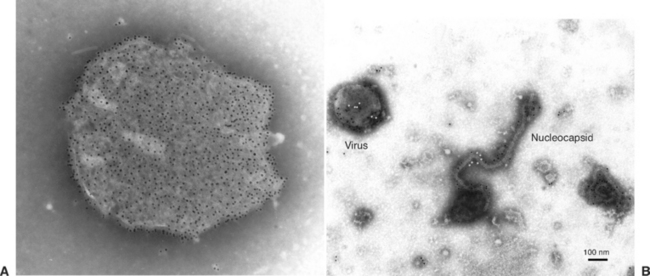
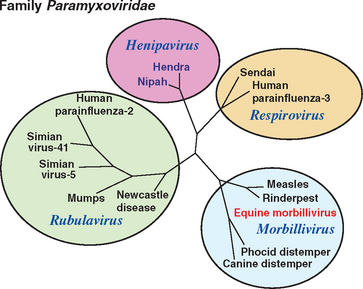
Hendra and Nipah viruses are the sole members of a new genus, Henipavirus, in the family Paramyxoviridae, subfamily Paramyxovirinae, order Mononegavirales. Virions are enveloped with a single-stranded, negative-sense ribonucleic acid (RNA) genome that is 18,234 nucleotides in length.35 In negatively stained electron micrographs, the virus is pleomorphic and approximately 180 nm in diameter. Unusually, the envelope is covered with two kinds of spikes that are 10 nm and 18 nm long and give the particle a “double-fringed” appearance (Fig. 16-3, A), which is not a feature of previously described paramyxoviruses. The nucleocapsid is 18 nm wide and has a periodicity of 5 nm36 (Fig. 16-3, B). Phylogenetic analyses confirmed that Hendra virus and Nipah viruses form a distinct clade within the family Paramyxoviridae37 (Fig. 16-4). Hendra virus does not agglutinate erythrocytes from a range of species, including human type O, monkey, equine, porcine, bovine, guinea pig, chicken, and goose, when tested at 4°, 22°, and 37° C, or possess detectable neuraminidase activity.
Epidemiology
Fruit bats (flying foxes) of the genus Pteropus, order Chiroptera, suborder Megachiroptera, are the natural hosts for Hendra virus. Antibodies were found in four Australian species of fruit bats, with up to 47% of the bats testing positive.38,39 Of 13 wildlife animal species tested, only bats had antibodies to Hendra virus.
Transmission of Hendra virus from bats to horses seems to be a rare event. The circumstances that facilitate transmission to and among horses remain unclear, but camping under trees or housing in buildings in which bats are roosting should be avoided. Evidence suggests that urine, aborted fetuses, and reproductive fluids from infected bats may be involved in transmission.38 After transmission to horses or humans, the virus does not appear to be highly contagious. Aerosol transmission among horses apparently is not a major mode of spread. In the original outbreak, a 5-km (3-mile) radius around the infected stables encompassed many other training stables, and the area was close to two major Thoroughbred racing and training tracks. Therefore the opportunity existed for many horses to become infected if the virus was readily transmissible. An extensive surveillance program undertaken in this area revealed no evidence of spread of infection beyond the two known, adjoining infected stables. No spread occurred to other stables contiguous with the two infected premises or to horses that shared the spelling paddock with the index case. Spread may have been inadvertently assisted by human actions. Serologic testing of more than 2500 equine sera collected from the outbreak area, a wider zone around Brisbane, and from other areas in Australia, in particular from horses with pneumonia, revealed no evidence of infection elsewhere.29
Pathogenesis
In horses, Hendra virus is predominantly pneumotropic but may also be neurotropic. All the equine cases and the first human case died from acute respiratory disease, whereas the second human patient died from encephalitis. Little is known about the portal of entry of the virus, the distribution and persistence of the virus in the body, or the routes of excretion of the virus. Horses were experimentally infected subcutaneously, intraocularly, and by aerosol, and virus was detected in nasal swabs collected postmortem. Virus was isolated from the lungs, liver, kidney, lymph nodes, and heparinized blood of experimentally infected horses. The virus was isolated from the kidney of the first human case, even though specific neutralizing antibody was detected in his serum.
Hendra virus attacks vascular endothelial cells and causes pulmonary edema. The lung and brain pathologies are somewhat similar to those produced by related viruses, such as canine distemper and measles. Experimentally, cats and guinea pigs are susceptible to Hendra virus, and although some pathologic differences exist in these two species, the diseases are similar to that observed in horses.40,41 Cats can be infected orally or parenterally and can transmit the virus to in-contact cats, although aerosol transmission of the virus was not demonstrated experimentally. There is no evidence of natural infection in cats, and serologic surveillance of cats in Brisbane yielded no evidence of feline infection. No infection or disease occurred in dogs, rats, mice, or chickens after experimental infection.42 There was infection but no disease in fruit bats after experimental infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree