CHAPTER 30 Miscellaneous Gram-Positive Bacterial Infections
CORYNEBACTERIUM PSEUDOTUBERCULOSIS
Sharon J. Spier and Mary Beth Whitcomb
Etiology
Corynebacterium pseudotuberculosis grows well at 36° C (96.8° F) on blood agar in 24 to 48 hours, and it forms small, pinpoint-diameter, whitish, opaque colonies surrounded by a weak zone of hemolysis. Because of the high content of lipids in the bacterial cell wall, particularly corynomycolic acid, the colonies spatter in a flame and can be pushed across the agar surface.1 The high content of lipids may facilitate survival of the organism in macrophages.2
Two species-specific biotypes of C. pseudotuberculosis have been identified based on differences in nitrate reduction,3 and deoxyribonucleic acid (DNA) fingerprinting techniques have revealed multiple strains.4–7 Biotypes isolated from small ruminants are nitrate negative, whereas those from horses are nitrate positive. From the result of DNA studies, the terms “biovar equi” for nitrate-positive and “biovar ovis” for nitrate-negative strains were proposed.3 Natural cross-species transmission does not seem to occur between sheep and horses; however, cattle can have infection from either biotype.3
Epidemiology
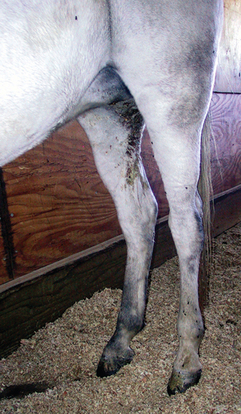
Three forms of C. pseudotuberculosis infection have been described in horses: ulcerative lymphangitis or limb infection, external abscesses (Fig. 30-1), and internal infection. Ulcerative lymphangitis presents as severe cellulitis, with involvement of lymphatic vessels in one or more limbs and multiple draining ulcerative lesions. In a retrospective study of C. pseudotuberculosis infection in horses from California, horses with ulcerative lymphangitis comprised only 1% of the cases, whereas external abscesses occurred in 91%, and internal abscesses in 8% of the cases.11 Horses of all breeds and gender may develop any of the three forms of disease, although mares may be overrepresented and Thoroughbreds underrepresented in studies by Doherr.16 In one recent study of internal infection, mares seemed to be overrepresented.20
The portal of entry of this soil-borne organism is thought to be through abrasions or wounds in the skin or mucous membranes. Many insects have been incriminated as vectors for the transmission of the disease to horses, and recent studies have shown that Haematobia irritans, Musca domestica, and Stomoxys calcitrans can act as vectors for this disease12 (Figs. 30-2 and 30-3). The regional location of abscesses suggests that ventral midline dermatitis predisposes to infection. Because of the variable incubation period, ventral midline dermatitis may not be present during maturation of the abscesses.
Temporal and spatial analysis suggests that the disease can be transmitted through horse-to-horse contact or from infected to susceptible horses by insects, other vectors, or contaminated soil; the incubation period is 3 to 4 weeks.13 The organism survives for up to 2 months in hay and shavings and more than 8 months in soil samples at environmental temperatures.14,15
The incidence of disease fluctuates considerably from year to year, presumably because of herd immunity and environmental factors such as rainfall and temperature. To date, the definitive environmental factors supporting the spread of infection have not been determined.16 Disease incidence is seasonal, with highest number of cases occurring during the dry months of the year, which is late summer and fall in the southwestern United States, although cases may be seen all year. Horses with internal infection are more frequently seen 1 to 2 months after the peak number of cases with external abscesses.17
Horses of all ages may be affected, although the low incidence of disease in foals less than 6 months of age suggests that passive transfer of immunoglobulins offers protection in foals born in endemic areas. A case-control study in an endemic area revealed young adults (<5 years) and horses in contact with other horses on summer pasture had increased risk of infection. Horses housed outside or with access to an outside paddock appeared to be at higher risk than stabled horses.18
Pathogenesis
The pathogenesis of C. pseudotuberculosis infection in horses is poorly understood. The incubation period appears variable; temporal and spatial clustering studies by Doherr et al.13 revealed an incubation period of 7 to 28 days. Bacteria are phagocytosed after entry into the host but continue to replicate. Intracellular survival of C. pseudotuberculosis has a key role in the formation of abscesses and is possibly mediated by two virulence factors: bacterial cell wall lipids and phospholipase D (PLD) protein exotoxin. The bacterial cell wall lipids may facilitate survival in macrophages, whereas the PLD exotoxin has profound effects on survival and multiplication within the host. The PLD toxin may directly affect phagocytic cells or inactivate complement and reduce opsonization of the bacteria. The exotoxin also increases vascular permeability, enhancing the spread of infection both locally and through the lymphatics. The subsequent vascular changes permit bacterial spread to additional locations, including regional lymph nodes. Humoral and cell-mediated immune responses ultimately develop, clearing the bacterial infection.19
Recovery generally is complete within 2 to 4 weeks, although rarely horses develop persistent or recurrent infections lasting for more than 1 year. In one study, 91% horses had complete recovery with no recurrence of infection in subsequent years, implying a long-lasting immunity.11 In 9% of affected horses, however, infections persisted more than 1 year or recurred as external or internal abscesses.11 In sheep and goats, studies have shown that acquired humoral and cellular immune responses develop after infection and that macrophages acquire the ability to kill the organism.19 Similar studies have not been performed in horses.
Corynebacterium pseudotuberculosis produces various exotoxins, which play a role in virulence; the most studied is PLD. Phospholipases are a heterogenous group of enzymes that share the ability to hydrolyze one or more ester linkages in glycerophospholipids. Although all phospholipases target phospholipids as substrates, each enzyme has the ability to cleave a specific ester bond. Thus, qualifying letters (e.g., A, B, C, D) are used to differentiate among phospholipases and to indicate the specific bond targeted in the phospholipid molecule. PLD is important in the pathogenesis of the disease by its action on cell membranes, causing hydrolysis and degradation of sphingomyelin in endothelial cells and increasing vascular permeability.8 The bacterial PLD is similar to the PLD of the brown recluse spider, which explains the presence of pain and edema at the site of infection. Targeted mutagenesis of PLD in C. pseudotuberculosis reduced the ability of this bacterium to establish a primary infection or cause chronic abscess formation in regional lymph nodes. These results indicate that PLD is a virulence determinant of C. pseudotuberculosis, increasing the persistence and spread of the bacteria within the host.9 The synergistic activity of C. pseudotuberculosis exotoxins with the exotoxins of Rhodococcus equi in lysing red blood cells in agar forms the basis for the synergistic hemolysis inhibition test.10
Clinical Findings
External Abscesses
External abscesses may occur anywhere on the body but most frequently develop in the pectoral region and along the ventral midline of the abdomen (see Fig. 30-1). This form of infection is commonly known as “pigeon fever,” because of the large size of the pectoral abscesses with the appearance of a pigeon’s breast, or “dryland distemper,” because of its prevalence in arid geographic regions. Abscesses contain tan, odor-free, purulent exudate and are usually well encapsulated. Additional sites with a predilection for abscess formation include the prepuce, mammary gland, axilla, triceps, limbs, and head. Other less common areas are the thorax, neck, parotid gland, guttural pouches, larynx, flanks, umbilicus, tail, and rectum. Septic joints and osteomyelitis have been reported.11 Horses may have an abscess involving a single site or involving multiple regions of the body. It is common to observe multiple subcutaneous abscesses coursing along a suspected lymphatic.
Clinical signs most frequently associated with external abscesses are edema, fever, and nonhealing wounds. Other clinical signs include lameness, ventral dermatitis, weight loss, depression, anorexia, and mammary gland or preputial swelling. Generally, horses with external abscesses do not develop signs of systemic illness, although one-quarter will develop fever.11 If signs of systemic illness are present, further diagnostic testing to rule out internal infection is warranted. In cases of external abscessation, a large area of edema is often observed in the area of abscess formation. As the abscess matures, this area becomes hard and painful. Some abscesses become quite large, particularly in the pectoral region. Abscesses typically have a thick capsule and can cause severe lameness if located in the axillary, triceps, or inguinal region.11 Maturation can be slow and drainage difficult to establish if the abscess lies deep to muscle.
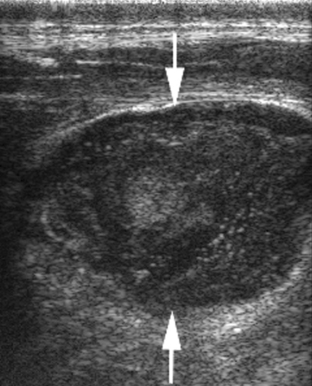
After drainage is established, either by spontaneous rupture or lancing, most horses recover within 10 to 14 days without complications. The abscesses may contain from 5 to 400 mL of thick, tan, purulent exudate. Ultrasonography may aid in determining the best location for drainage of external abscesses (Fig. 30-4). The case fatality for horses with external abscesses is very low (0.8%).11
Internal Infection
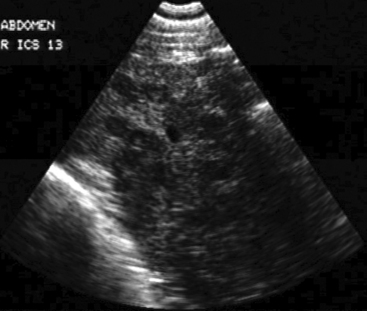
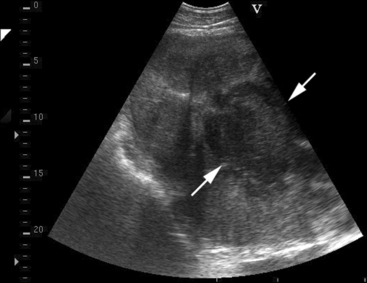
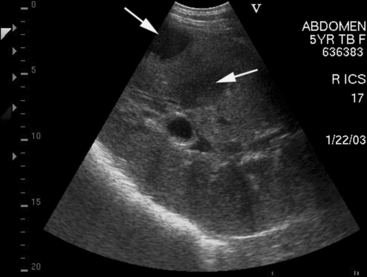
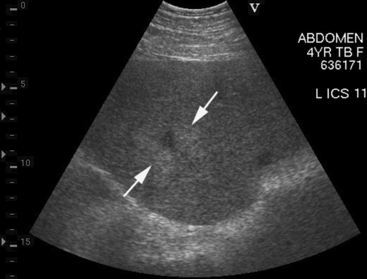
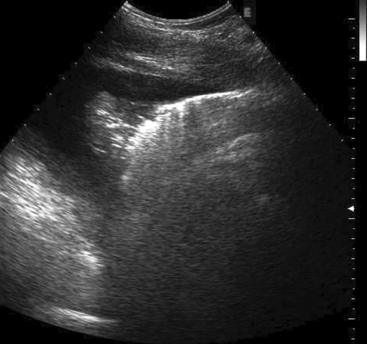
Approximately 8% of affected horses develop internal infection, which is associated with a case-fatality rate of 30% to 40%.11 In a retrospective study, infection was localized to a specific organ(s) in 90% (27/30) of horses. Involvement of multiple internal organs was identified in 37% (10/27) of horses. The organs most often involved were liver and lungs, with kidney and spleen being affected less often. Abdominal ultrasonography was a useful diagnostic tool to identify affected abdominal organs specifically17,20 (Figs. 30-5 to 30-8).
A diagnosis of internal infection is based on clinical signs, clinicopathologic data, serology, diagnostic imaging, and bacterial culture. The most common clinical signs are concurrent external abscesses, decreased appetite, fever, lethargy, weight loss, and signs of respiratory disease or abdominal pain. Other signs observed in horses with internal abscesses include ventral edema, ventral dermatitis, ataxia, hematuria (caused by renal abscesses), and infrequently, abortion. The median age in two studies was 7 and 8 years, with a range of 1 to 23 years.11,20
Anemia of chronic disease, leukocytosis with neutrophilia, and elevated fibrinogen are common features of infection, particularly in horses with internal abscesses11 (Table 30-1). Leukocytosis with neutrophilia was seen in 36% and 76% of horses with external and internal abscesses, respectively. Hyperproteinemia, caused by increased serum globulin concentrations, was observed in 38% and 59% of horses with external and internal abscesses, respectively.11
Table 30-1 Values of Key Clinicopathologic Data in 30 Horses with Internal Corynebacterium pseudotuberculosis Infection
| CLINICOPATHOLOGIC DATA | MEAN ± SD | RANGE |
|---|---|---|
| Hematocrit (%) | 33.9 ± 7.2 | 23.4–50.0 |
| Total white blood cell (WBC) count (cells/μL) | 19,297 ± 9485 | 6400–53,420 |
| Neutrophil count (cells/μL) | 16,080 ± 9109 | 2944–49,627 |
| Platelet count (cells/μL) | 228,967 ± 86,280 | 15,000-449,000 |
| Fibrinogen (mg/dL) | 670.0 ± 306.4 | 100–1600 |
| Total protein concentration (g/dL) | 8.7 ± 1.5 | 6.3–12.1 |
| Globulin concentration (g/dL) | 6.7 ± 1.8 | 3.5–10.5 |
| Synergistic hemolysis inhibition (SHI) titer | 2611 | 64–20,480 |
Data from Pratt SM, Spier SJ, Vaughan B, et al: J Am Vet Med Assoc 227:441-448, 2005.
Peritoneal fluid is frequently abnormal in horses with abdominal abscesses. However, peritoneal fluid analysis may be normal if abscesses are located retroperitoneal in the kidneys without involvement of other abdominal structures. C. pseudotuberculosis was isolated from 32% of samples of peritoneal fluid from affected horses.11 Failure to isolate the organism from peritoneal fluid does not rule out the disease. The organisms could be located retroperitoneal, sequestered within a thick capsule, or suppressed by local factors or nucleated cells.
Ulcerative Lymphangitis
Ulcerative lymphangitis is the least common form of C. pseudotuberculosis seen in horses in North America, although this form of disease has been reported worldwide. Limb swelling, cellulitis, and draining tracts following lymphatic vessels are seen. Horses often develop severe lameness, fever, lethargy, and anorexia. Aggressive medical therapy is necessary, or the disease may become chronic, resulting in limb edema, lameness, weakness, and weight loss.1
Diagnosis
Culture
The typical clinical presentation of single or multiple, maturing pectoral abscesses, with or without ventral midline abscesses, is highly suspicious of C. pseudotuberculosis infection. Culture of the characteristic tan or blood-tinged, odorless exudate is diagnostic. Bacteriologic culture of aspirates or draining abscesses readily yields growth of moderate to large numbers of organisms on blood agar in 24 to 48 hours. The colonies appear small, white, and opaque. Gram stain reveals gram-positive pleomorphic rods. Equine isolates reduce nitrate (unlike strains from small ruminants). In horses with internal infection, samples obtained by ultrasound-guided aspiration from affected organs can also yield a positive culture.17–20
Serology
Without a positive bacterial culture, the practitioner must rely on hematology, clinical chemistry, and serologic testing to support a diagnosis. Hematologic changes are nonspecific and indicative of a chronic inflammatory response. Serologic testing using the synergistic hemolysis inhibition (SHI) test can be a useful aid in the diagnosis of internal abscesses.10,11 The SHI test measures immunoglobulin G (IgG) to the exotoxin of C. pseudotuberculosis and is available through the California Animal Health and Food Safety Laboratory System in Davis, California. Serology is generally not helpful for diagnosis of external abscesses and may be negative early in the course of disease and even at the time of abscess drainage. Positive SHI titers must be interpreted carefully, after appropriate consideration of clinical signs, to distinguish active infection from exposure or convalescence. Both published and unpublished data from the University of California suggest that a reciprocal titer of 256 or greater is indicative of active infection. Horses with internal abscesses generally have SHI titers of 512 or higher. In one study of internal infection, SHI titers ranged from 512 to 20,480.20 Titers of 16 or lower are considered negative, whereas titers between 16 and 128 are considered suspicious or indicative of exposure.21 These are only general guidelines, however, because there is considerable overlap in results from horses with active disease, exposure, and recovery from infection. Experimental inoculation of horses with a bacterin-toxoid developed from C. pseudotuberculosis produced increased SHI titers equivalent to those seen with active infection; the protection offered from this bacterin has not been proved to date.
Ultrasonography
Ultrasonography is extremely useful for diagnosis of internal infections in horses, not only for identifying affected organs, but also for defining the nature and extent of involvement of abdominal viscera. Abdominal ultrasound permits documentation of involvement of specific organs in the absence of clinicopathologic evidence of disease involving these organs. Abdominal ultrasound may be used for collection of transcutaneous liver and kidney biopsies and aspirates of abscesses for a definitive diagnosis (see Fig. 30-8). Thoracic ultrasound should be performed in affected horses to determine the severity of pulmonary disease (Fig. 30-9). Serial examinations can be used to evaluate response to treatment. Examinations should be performed using low-frequency (2.5-3.5 MHz) and medium-frequency (5 MHz) ultrasound transducers. Complete examination of the abdomen consists of both right and left paralumbar fossa regions, all intercostal spaces from the ventral lung margins to the costochondral junctions, and the ventral abdomen from the sternum to the inguinal region. Presence of peritoneal fluid and abnormalities of the liver, spleen, kidneys, stomach, duodenum, small intestine, cecum, and large colon can be observed.
Hepatic abnormalities associated with C. pseudotuberculosis infection in horses include hepatomegaly, multiple small hypoechoic areas resulting in a “moth-eaten” appearance, and discrete, circular, anechoic to hypoechoic areas (see Fig. 30-5). Renal abscessation may appear as a single large (10-15 cm in diameter) area or multiple anechoic to hypoechoic areas involving either the cortex or the medulla17 (see Figs. 30-6 and 30-7). Splenic abnormalities may also be observed and include small, irregularly shaped, hypoechoic areas without obvious encapsulation (see Fig. 30-8). In horses with pulmonary involvement, examination of the thorax may reveal presence of pleural defects (“comet tails”), consolidation, pleural effusion, or pericardial effusion (see Fig. 30-9).20
Early diagnosis of internal infection caused by C. pseudotuberculosis is important for a successful outcome but is often difficult because of the insidious onset and nonspecific nature of clinical signs, which may include anorexia, fever, lethargy, and weight loss. In endemic areas, horses that have had an external abscess in the previous 6 months and then develop signs of systemic illness should be suspected of having internal C. pseudotuberculosis infection, as should horses with compatible signs residing on a property where other horses have had external abscesses. For horses with this history, ultrasonography and serologic testing are recommended as an aid for diagnosis of internal infections.
Therapy
External Abscesses
The treatment regimen must be individualized for each horse depending on the severity of disease, including the presence of systemic illness (e.g., fever, anorexia), the extent of soft tissue inflammation, the maturity of the abscess, and the ability to establish drainage of pus. Establishing drainage is the most important treatment and ultimately leads to faster resolution and return to athletic performance. The proximity of the fibrous abscess capsule to the skin varies, often being less than 1 cm deep for ventral midline abscesses, to greater than 10 cm for deep pectoral, axillary, triceps, or inguinal abscesses (Fig. 30-10). Aspiration and drainage of superficial abscesses are easily performed; the use of diagnostic ultrasound is helpful for localization of deeper abscesses and to judge maturity of the abscess and proximity to the skin. If the abscess is immature or cannot be safely incised, subsequent ultrasound examinations may be necessary to establish the ideal time to lance into the abscess. The abscess contents and lavage solutions (e.g., saline with or without antiseptic) should be retrieved and disposed of to prevent further contamination of the immediate environment.
Antimicrobial therapy is indicated for treatment of horses with ulcerative lymphangitis or internal abscesses. The use of antimicrobials for external abscesses is not necessary in most horses and may prolong the time to resolution.11 Antimicrobial therapy may be justified when signs of systemic illness (e.g., fever, depression, anorexia) or extensive cellulitis are present. Horses with deep intramuscular abscesses that are lanced and draining through healthy tissue may also benefit from antimicrobial therapy.
Corynebacterium pseudotuberculosis is susceptible in vitro to many antimicrobials typically used in horses, including penicillin G, macrolides, tetracyclines, cephalosporins, chloramphenicol, fluoroquinolones, and rifampin, but some isolates may be resistant to aminoglycosides.7,22,23 Several factors should be considered when choosing an antimicrobial: the intracellular location of the organism, the presence of exudate and a thick abscess capsule, anticipated duration of therapy, cost of the drug, and convenience of administration. Despite in vitro susceptibility, the nature of the bacteria and the copious exudate render certain antimicrobials ineffective for some cases. Trimethoprim-sulfa (5 mg/kg based on the trimethoprim fraction, twice daily orally) and procaine penicillin (20,000 U/kg twice daily intramuscularly) are highly effective for treatment of external abscesses, especially on the ventral midline. Rifampin (2.5-5 mg/kg twice daily orally) in combination with ceftiofur (2.5-5 mg/kg twice daily intravenously or intramuscularly) appears highly effective for treatment of internal abscesses. Internal abscesses have reportedly responded to procaine penicillin (dose as above), trimethoprim-sulfa (dose as above), potassium penicillin (20,000-40,000 U/kg four times daily intravenously), as well as erythromycin estolate (15-25 mg/kg twice daily orally).
Internal Infection
Antimicrobial therapy is indicated for the treatment of horses with systemic infection caused by C. pseudotuberculosis.11 The median duration of antimicrobial therapy in a recent study was 36 days and ranged from 7 to 97 days. A variety of antimicrobials to which C. pseudotuberculosis is susceptible were used to treat these internal infections (Table 30-2). Rifampin was used in combination with another antimicrobial in the majority of horses. Rifampin alone was used for continued treatment in horses after initial treatment with a combination of rifampin and another antimicrobial.18 Thoracic and abdominal ultrasound are useful to monitor response to therapy. Ultrasound findings, in addition to clinicopathologic data, aid in the decision-making process for continued antimicrobial therapy in these cases.
Table 30-2 Antimicrobial Drugs Used to Treat 30 Horses with Internal C. pseudotuberculosis Infection
| ANTIMICROBIAL | NO. OF HORSES | NO. ALSO WITH RIFAMPIN |
|---|---|---|
| Rifampin | 19 | — |
| Trimethoprim-sulfa | 11 | 3 |
| Gentamicin | 10 | — |
| Ampicillin | 9 | 4 |
| Ceftiofur | 9 | 7 |
| Penicillin G | 5 | 3 |
| Cefazolin | 2 | — |
| Enrofloxacin | 2 | 2 |
| Erythromycin | 2 | — |
Data from Pratt SM, Spier SJ, Vaughan B, et al: J Am Vet Med Assoc 227:441-448, 2005.
The overall mortality associated with internal infections is reported to be 30% to 40%,11,20 but horses that did not receive antimicrobial therapy had a 100% fatality rate.11 Antimicrobial therapy for treatment of internal abscesses and ulcerative lymphangitis must be continued for 1 to 6 months. Resolution of infection is determined based on clinical signs, normal clinical pathologic values, and decline in immunoglobulin concentrations. Some horses with very high SHI titers remain seropositive for up to 1 year because of the lengthy half-life of IgG (21 days) and for other reasons that are unknown. Under such situations the clinician should monitor a steady decline in serum SHI titers to C. pseudotuberculosis. Purpura hemorrhagica or vasculitis has been reported in horses with systemic infection requiring concurrent therapy with antimicrobials and corticosteroids.11
Ulcerative Lymphangitis
Horses with ulcerative lymphangitis or cellulitis should be treated early and aggressively with antimicrobials, or residual lameness or limb swelling may occur. Typically, intravenous (IV) antimicrobials (ceftiofur or penicillin G) alone or in combination with rifampin (orally) are used until lameness and swelling improves, and then therapy with oral antimicrobials such as trimethoprim-sulfamethoxazole or rifampin is continued to prevent relapse. The time to resolution in one study was approximately 35 days.11 Physical therapy, including hydrotherapy, hand walking, and leg wraps, as well as administration of nonsteroidal antiinflammatory drugs (NSAIDs), are also recommended.
Prevention
A commercial bacterin-toxoid is available for use in small ruminants (Caseous D-T, Colorado Serum Co., Denver; Glanvac 6 TM, Pfizer Animal Health, Australia).24 The safety and effectiveness of this product has not been tested in horses. The inability experimentally to reproduce the disease as seen in horses in endemic areas and the sporadic nature of disease complicate research efforts.
NOCARDIOSIS
Nocardiosis is a localized or disseminated bacterial disease of a large variety of animals and of humans caused by Nocardia spp.,25–28 which are soil saprophytes that act as opportunistic pathogens.25,29 In humans and domestic animals, Nocardia causes suppurative to granulomatous tissue reactions, which are most severe in immunosuppressed individuals.30 Nocardia infections in horses are rare. When they do occur, they are usually associated with significant derangements in the host immune system.30–32 The most common manifestations of disease are pulmonary and pleural disease or nodulo-ulcerative cutaneous lesions.
Etiology
Members of the Nocardia species are gram-positive, aerobic, saprophytic, nonmotile, non–spore-forming actinomycetes.25,27,28,33,34 Nocardiae appear as long, slender, branching filaments with a tendency to fragment into rods and cocci.34 When cultured, these organisms produce aerial filaments.27,34 Components of the cell wall, especially mycolic acid, render Nocardia species partially acid-fast.24,27,28,34
Although most nocardial infections in horses are caused by Nocardia asteroides,27,29,30,34 Deem and Harrington26 reported a case of fatal pleuropneumonia in a 15-month-old Quarter Horse colt with histologic lesions characteristic of pulmonary nocardiosis in which Nocardia brasiliensis was isolated from the lung and bronchial lymph node.
Pathogenic nocardiae are obligate aerobes growing over a wide temperature range (10°–50° C [50°–122° F]).24 Nocardia spp. will grow on most nonselective media used routinely for culture of bacteria, fungi, and mycobacteria.28,33,37 However, in specimens containing mixed flora (e.g., respiratory secretions), nocardial colonies are easily obscured by those of more rapidly growing bacteria. Nocardiae normally appear within 2 to 7 days on most routine bacteriologic media.37,38 Their relatively slow growth often results in the cultures being discarded before the nocardiae can be visualized.27,37 The colony surface, waxy to powdery to velvety depending on the abundance of aerial growth, becomes wrinkled with age.25,33,37,43 Although the colonies are usually white when first visible, most Nocardia spp. produce carotenoid-like pigments that result in colonies with various shades of yellow, orange, pink, or red.25,27,43
With the widespread use of molecular methods of identification, the number of recognized Nocardia spp. is increasing, and members of the genus Nocardia are becoming increasingly difficult to identify using phenotypic criteria.35 Currently, more than 30 species are included in the genus.25,36
Molecular analysis demonstrates that N. asteroides includes several species with similar biochemistry, structure, and antimicrobial resistance: N. asteroides, N. abscessus, N. cyriacigeorgica, N. farcinica, N. nova, and N. transvalensis.25,28,36–38 These species together are referred to as the N. asteroides complex.25,36–38 It is important to note that all the literature about nocardiosis in horses was published before these species were recognized, and therefore it is impossible to determine the clinical relevance of each individual species in horses. For purposes of simplicity, the discussion that follows will use the former name of N. asteroides.
Epidemiology
Nocardiae are present in most environments and found extensively worldwide. They are saprophytic, making up an important component of the normal soil microflora, and are often associated with water.25,33,38 Nocardial infections may be acquired through inhalation27,33,34,38 or by traumatic percutaneous introduction of organisms.33,38 Dust, soil, and plant material serve as vehicles.25,27 In humans, intestinal nocardiosis may result from ingestion of the organism;27,34 however, this form of infection has not been reported in horses. Infection is not considered contagious.33 Transmission between or among animals and persons has not been demonstrated to occur.26,37,38 Nocardiosis is not considered a zoonotic disease.33
In the horse, pulmonary and generalized infection appears to require profound constitutional and immune disturbances and should be considered an opportunistic infection.25,31,34 In a review of 16 horses with nocardiosis admitted to the Veterinary Teaching Hospital, University of California, Davis, over an 18-year period, almost 90% had an underlying immunosuppressive problem.31 The two horses that survived had local infections associated with trauma. The remaining 14 cases were associated with concurrent serious systemic illnesses and compromised immune systems that would have led to the death of the horses in the absence of nocardiosis. From the same study, the prevalence of nocardiosis in equine patients from 1965 to 1983 was 16 of 180,000 (0.009%). Two entities emerged as predominant conditions linked with systemic or pulmonary nocardiosis in horses: severe combined immunodeficiency of Arabian foals and pituitary pars intermedia dysfunction.31 It appears that the circumstances favoring nocardiosis infection in horses closely parallel those recognized in human medicine, where the majority of patients with clinically recognized Nocardia asteroides infection have underlying debilitating and/or immunodepressant conditions.25,26,31,38–40