CHAPTER 57 Miscellaneous Fungal Diseases
BLASTOMYCOSIS
Etiology
The thermally dimorphic ascomycete Blastomyces dermatitidis is the cause of all manifestations of blastomycosis. Conidia, measuring 2 to 10 μm in diameter, are the infectious agents. The yeast form is thick walled and usually 8 to 15 μm in diameter, although yeasts as large as 30 μm have been reported.1 In tissues, yeast cells display characteristic broad-based buds.
Virulence Factors
Several factors contribute to the virulence of B. dermatitidis.2 In tissues the organism converts to a yeast form that is more resistant to phagocytosis and killing than are conidia. The yeast cell wall may have antiphagocytic properties, and cell wall phospholipids may contribute to virulence.3 The BAD1 antigen (formerly called WI-1), a cell wall surface antigen, is an important factor for the adherence of yeasts to macrophages and to lung tissue. Loss of the antigen results in impaired binding and entry into macrophages and in diminished capacity of the yeast to adhere to the lung. A strain of B. dermatitidis experimentally deprived of this antigen by gene deletion was avirulent in mice.4 BAD1 has other antiinflammatory effects that include influencing the profile of proinflammatory cytokines, such as suppressing tumor necrosis factor alpha (TNF-α) release by host macrophages.5
Epidemiology
The ecology of the filamentous form of B. dermatitidis in nature has proved challenging to define, and the organism is difficult to isolate from environmental sources. Based on environmental isolates in two outbreaks of human and canine disease, the mycelial form of B. dermatitidis is thought to be a saprophyte found in moist soil of acid pH and high organic content, usually in wooded areas near water.1,2 Optimal conditions for growth of the mycelia in its natural microenvironment may be short lived, and the organism may be present in large numbers only transiently. B. dermatitidis is endemic in the Mississippi, Missouri, and Ohio River watersheds, the Great Lakes regions of the United States and Canada, and the St. Lawrence River Valley1,2 (Fig. 57-1). The disease is also found in Africa, but is seldom reported from other parts of the world.
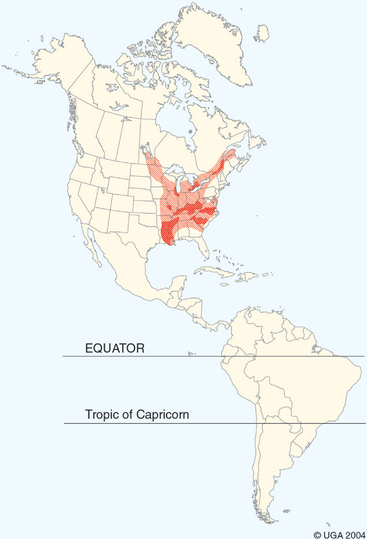
Fig. 57-1 Shading indicates area of endemic blastomycosis. Darker shading indicates areas of higher incidence.
(From Greene C: Infectious diseases of the dog and cat, ed 3, Philadelphia, 2006, Saunders.)
Blastomycosis has been reported in many species, most often in dogs and humans,6 and rarely in horses. Dogs are considered sentinel animals for human infections. Blastomycosis is not contagious or zoonotic among infected persons or animals. The disease occurs sporadically, although clusters of cases have been described and are likely associated with an increase in infective conidia in an environment that is transiently optimal for mycelia growth. B. dermatitidis was isolated from soil on the banks of a beaver pond near a camp in Wisconsin; 48 people who visited the camp developed blastomycosis.7 Most infections occur during cooler and wetter months of the year. Agricultural workers, hunters, campers, and their canine companions are particularly at risk for infection.1
The prevalence of asymptomatic pulmonary infections is unknown. Spontaneous recovery from an identified blastomycosis infection in humans is uncommon, and mortality rates in untreated persons with chronic blastomycosis may be as high as 60%.2 B. dermatitidis organisms are not among the flora of healthy persons. These findings suggest that the incidence of asymptomatic infection may be low.
Pathogenesis
Blastomycosis is a disease of immunocompetent persons and is relatively infrequently reported as an opportunistic infection in immunocompromised patients.2 In patients with acquired immunodeficiency syndrome (AIDS), the neurologic form of blastomycosis is more common, and the disease is more severe and more often fatal than in immunocompetent patients.8 Infection most often occurs by inhalation of aerosolized conidia. In the lungs the conidia are phagocytized by neutrophils, monocytes, and alveolar macrophages. Macrophages may inhibit the conversion of conidia to yeasts, but conidia that remain viable in tissues rapidly transform into the more resistant yeast. A robust cellular immune response is crucial in the host’s defense against the organism. B. dermatitidis has a predilection for bone and skin and may reach these organs by dissemination from the lungs. Infection may also occur by direct inoculation through the skin.
Intravenous inoculation of one horse with B. dermatitidis caused chronic inflammatory foci in the lungs.9 Subcutaneous inoculation did not produce disease. No further details of these studies are available.
Clinical Findings
Clinical signs in human patients with blastomycosis are variable and often nonspecific; weight loss, fever, malaise, and fatigue are common to many diseases. The onset is usually insidious, and the clinical course is often chronic. Pulmonary, cutaneous, osseous, genitourinary, and central nervous system abnormalities, as well as sporadic disease of almost any organ, have been reported in humans.2 Forty percent of dogs with blastomycosis have ocular lesions.6 Misdiagnosis is common in human patients, possibly because the index of suspicion for this disease is low, but also because blastomycosis can mimic many other diseases. For example, pulmonary manifestations of blastomycosis can be easily confused with bacterial pneumonia, tuberculosis, or neoplasia.2
Three equine cases have been reported in the literature,10–12 and the author has seen one additional case. Two horses had cutaneous manifestations of blastomycosis. One had chronic subcutaneous abscesses, initially in the perianal and perivulvar region and progressing over several months to involve the perineum, udder, and ventral midline.10 Multiple abscesses were noted, and abscess maturation, spontaneous decompression, resolution, and appearance of new abscesses became a constant cycle as the disease steadily worsened. A partial necropsy showed that the inflammatory process was locally aggressive. The diagnosis was based on the histopathologic identification of the typical yeasts in tissue sections showing associated pyogranulomatous inflammation. Fungal cultures were not attempted.
A miniature horse had a 6-month history of chronic subcutaneous infections in the cervical and pectoral regions, complicated by acute onset of dysphagia.12 An open, draining wound communicated with a tract extending toward the caudal deep cervical lymph nodes; a mediastinal mass identified by ultrasonography appeared to connect by a tract to the nodes. There were abscesses in the right lung, and a bilateral pleural effusion was present. At necropsy, pyogranulomatous inflammation was found in the left kidney and the right lung. Multiple pleural abscesses and peritonitis were also present.
Two horses have had fatal disseminated blastomycosis without cutaneous disease.11,13 Lethargy, weight loss, and cough were historical clinical signs. Both horses had pyogranulomatous pleuritis and peritonitis. Lung, mediastinum, liver, squamous stomach, large intestinal serosa, and diaphragm were affected in one horse,13 whereas most abdominal viscera and the thoracic cavity were affected in the other.11 In both horses, adhesions of pulmonary abscesses to the diaphragm were associated with disease on the corresponding abdominal surface of the diaphragm, suggesting extension of the inflammation from one body cavity to the other. In these cases, typical yeasts were seen in peritoneal and pleural fluid antemortem and in tissues at necropsy. B. dermatitidis was identified in culture, and in one horse the identification of the organisms in culture was confirmed by molecular techniques.11
In this small case series, blastomycosis in horses tended to be a disseminated or locally aggressive and fatal disease. Two of the affected horses resided in the Ohio River Valley (a region where blastomycosis is endemic), and a third was from eastern Pennsylvania. Although rare, blastomycosis should be considered a differential diagnosis for horses with pneumonia, pleuritis, peritonitis, or skin disease that is poorly responsive to antibiotic therapy, particularly if the affected horse resides in a region where blastomycosis is endemic.
Diagnosis
Cytology
Cytologic examination of body fluids (transtracheal aspirate, bronchoalveolar lavage, pleural, peritoneal) often allows identification of typical, thick-walled yeasts that are 8 to 15 μm in diameter with broad-based budding.1 The sensitivity of cytologic examination for diagnosis of blastomycosis in human patients was 93% in one study.14
Biopsy
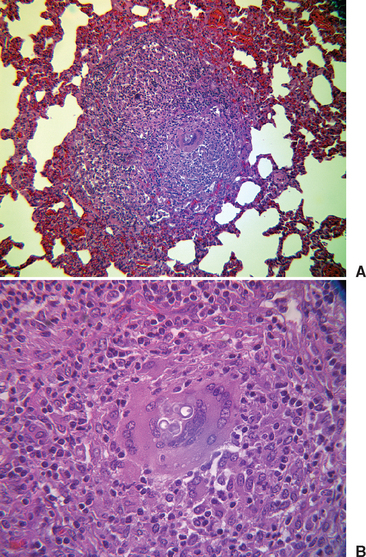
In biopsies of tissues from affected humans, epithelioid granulomata, pyogranulomatous inflammation, necrosis, and fibrosis may be seen.1 Yeasts are usually plentiful in infected tissues (Figs. 57-2 and 57-3). The thick-walled yeast cells are generally of uniform size (8-15 μm in diameter), although large cells (20-30 μm) have been described. Broad-based budding is the identifying feature and must be seen to have strong evidence of the presence of B. dermatitidis. The yeast wall may not stain with hematoxylin and eosin (H&E), and the cytoplasm may shrink away from the wall, leaving a space.1 The sensitivity of histopathologic testing for the diagnosis of blastomycosis in persons in one study was 81.5%.14
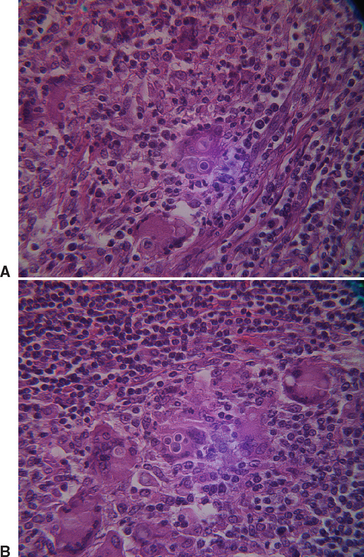
Fig. 57-2 Photomicrographs of Blastomyces dermatitidis in lymph node of the horse described by Toribio et al.11 A, Note broad-based bud. B, Note multiple giant cells.
Culture
Samples of infected body fluids or tissues usually produce characteristic yeasts within 2 to 4 weeks in cultures incubated at 37° C (98.6° F).2 Culture is still considered the “gold standard” for the diagnosis of blastomycosis in human patients, although the sensitivity of this test in one study was 66.4%.14
On Sabouraud dextrose agar incubated at 25° C (77° F), the growth rate of the filamentous fungi is variable and slow, and cultures should be held for 2 months or more.1 Colonies may be flat and glabrous (described as “skinlike”) or may be fluffy white to brownish tan, sometimes with concentric rings. The appearance of the conidia is not distinctive, and identification of B. dermatitidis depends on observing the characteristic yeasts that appear within days to weeks when subcultures are incubated at 37° C.1
Molecular Identification of Yeasts
Molecular techniques for identification of the yeast form of B. dermatitidis in culture and fixed tissues are commercially available.15 The application of deoxyribonucleic acid (DNA) amplification techniques to samples of body fluids or fresh tissues from clinical patients requires further study.
Serology
Antibody Detection.
Serology has not proved to be a sensitive or specific method of diagnosing blastomycosis in humans. Immunocompromised persons may produce low concentrations of antibodies. A comparison of results from enzyme-linked immunosorbent assay (ELISA), complement fixation (CF), and immunodiffusion (ID) tests for antibody to B. dermatitidis in persons with documented blastomycosis showed that the assays had diagnostic sensitivity of 80%, 40%, and 65%, respectively.16 Cross-reactions with other fungi (particularly Histoplasma capsulatum) are common.2 An agar-gel immunodiffusion (AGID) test currently used in the diagnosis of canine blastomycosis has a sensitivity and specificity of greater than 90%. However, positive serologic testing alone is not considered sufficient to establish the diagnosis.6
When purified WI-1 (BAD1) antigen was used in a radioimmunoassay (RIA) to detect serum antibodies to B. dermatitidis, 85% of 68 persons known to have the disease were identified.17 The RIA also detected 3% of patients with histoplasmosis, coccidioidomycosis, sporotrichosis, or candidiasis. All healthy persons had negative tests. In another study, anti–WI-1 serologic testing identified 75% of 32 human patients with blastomycosis.18 Testing by RIA detected anti–WI-1 antibodies in 92% of infected dogs,19 and RIA titers declined during treatment, suggesting that the RIA might be useful in monitoring the progress of therapy in affected dogs. AGID detected antibodies against the A antigen of B. dermatitidis in 41% of dogs with blastomycosis.19
Antigen Detection.
Detection of B. dermatitidis antigen in the urine of persons and dogs with blastomycosis has been reported. Durkin et al.14 used antibodies to mold antigens in an immunoassay and showed that the sensitivity of the test in patients with blastomycosis was 92.9% and the specificity 79.3%. Cross-reactions occurred in 96% of patients with histoplasmosis, 100% of those with paracoccidioidomycosis, 2.9% of those with cryptococcosis, and 1.1% of patients with aspergillosis. Shurley et al.19a used a competitive-binding inhibition ELISA test with antibodies raised against yeast-phase lysates and detected antigen in the urine of 100% of 36 dogs with blastomycosis. Unfortunately, cross-reactivity with H. capsulatum and elements in urine from healthy dogs were observed in this study. The sensitivity and specificity of tests for antigen determination should be optimized before these assays can be used reliably in the clinical setting. These tests may be useful in combination with results of physical examination, cytology, and culture in patients with suspected blastomycosis.
Therapy
Antifungal treatment of horses with blastomycosis has not been attempted.
Recommendations for treatment of humans with blastomycosis are based on limited data.2 Amphotericin B (AMB) (0.5-0.6 mg/kg/day until the disease is controlled) is the treatment of choice in patients with life-threatening disease. After the disease has been controlled, an oral azole drug can be substituted for AMB. Itraconazole is the drug of choice for patients who have non–life-threatening disease that does not involve the central nervous system (CNS). Ketoconazole can be used for pulmonary infections that are not life threatening, although there are reports of CNS blastomycosis in patients who were successfully treated with ketoconazole for chest disease.2 Ketoconazole distributes poorly to the CNS. Fluconazole is reported to have efficacy comparable to ketoconazole, is less toxic, and achieves good concentrations in the CNS. Fluconazole is an alternative in patients who cannot tolerate itraconazole.
Prevention
Recent studies have demonstrated that mice can be protected from an intrapulmonary challenge of B. dermatitidis by subcutaneous injection of a mutant strain of the fungus.5 The mutant strain lacks the BAD1 gene and therefore the BAD1 cell surface antigen, previously shown to be a virulence factor. Mouse protection was characterized by the induction of a strong cell-mediated immune response.
CRYPTOCOCCOSIS
Etiology
Cryptococcus neoformans and Cryptococcus gattii, formerly known as C. neoformans var. gattii,20 are the cause of virtually all cryptococcal disease in mammals.21 Because of the recent change in classification of C. gattii, most of the literature refers to this organism as a variant of C. neoformans. Consequently, in this chapter, unless otherwise stated, comments regarding Cryptococcus neoformans can be taken to refer also to C. gattii. C. neoformans, classified in the phylum Basidiomycotina, is a spherical to ovoid, budding yeast 3 to 25 μm in diameter22 that possesses a capsule 1 to 30 μm in thickness composed of at least three heteroglycans.23 The heteroglycan composition of the capsule is the basis for distinguishing the many species of C. neoformans, of which two (in addition to C. gattii) are the cause of most diseases: C. neoformans var. grubii and C. neoformans var. neoformans. In animal tissues and fluids the yeast reproduces by typically narrow-based budding.23
Virulence Factors
Cryptococcus neoformans has three genetically controlled characteristics largely responsible for its virulence: the ability to produce a capsule, melanin formation, and the ability to grow at 37° C.24
Capsule.
The distinctive polysaccharide capsule of the yeast affords several modes of protection from the host’s immune response. The capsule blocks phagocytosis, masks ligands on the fungal cell surface to which antibodies might bind, reduces the respiratory burst of phagocytes, downregulates production of protective cytokines (T helper cell type 1 [Th1] response), and upregulates production of Th2 cytokines.21,24 Mutant cryptococcal organisms that have deficient capsules are less virulent than their parent strains, and increases in virulence have been associated with structural changes or increased thickness of the capsule.24,25 The capsules of the yeast may enlarge during infection.24,26 Circulating solubilized capsular polysaccharide antigens in human patients with disseminated cryptococcosis may inhibit leukocyte migration to the site of cryptococcal infection.27
Melanin.
Cryptococcus organisms are efficient producers of melanin through the enzyme phenol oxidase.25 Melanin scavenges oxygen free radicals derived from leukocytes, and this antioxidant effect contributes to virulence.24,27 Melanin also impairs antibody formation, depresses lymphoproliferation, and downregulates TNF-α production.21 Because it contributes to the negative charge of C. neoformans, melanin may also impair phagocytosis of the organism.27
Thermotolerance.
The ability to live and replicate at mammalian body temperature (37° C) is a characteristic of invasive fungal pathogens in general. Temperature-sensitive mutant strains of C. neoformans are avirulent in mammalian models of cryptococcal disease. The ability to grow at high temperature is under the control of multiple genes in C. neoformans.24
Epidemiology
Cryptococcus neoformans is an opportunistic, saprophytic yeast that is ubiquitous worldwide. Bird manure, particularly pigeon droppings, and bat guano harbor C. neoformans var. grubii and C. neoformans var. neoformans, which can remain viable for up to 2 years in fecal material.1 Purines, urea, and creatinine are present in high concentrations in bird excreta and readily used by the yeast as substrates for metabolism.28,29 The pigeon may be the most important vector for dispersing C. neoformans and maintaining it in the environment.1 C. neoformans is also found in rotting wood, and C. gattii has been associated with flowering red and river gum trees (species of Eucalyptus trees) in Australia, Columbia, India, and southern California.23 The prevalence of eucalyptus trees in the environment may explain the relatively frequent occurrence of cryptococcosis in horses and other species in western Australia.30,31 C. gattii is endemic in Australia.20 Admixture of soil with contaminated feces or rotting material from trees results in a reduction in numbers of organisms, possibly because of ingestion by soil amebae.1 The yeast can also be isolated from ripe fruits, rotting vegetables, and dairy products.1 High humidity fosters growth of C. neoformans, whereas exposure to direct sunlight can kill the yeast.1
Disease caused by C. neoformans usually occurs sporadically. Outbreaks comprising multiple cases of affected persons or animals, presumably exposed to the same source of the organism, are infrequently reported, and transmission of disease among affected animals or humans has not been described.25 A large outbreak of C. gattii in persons, dogs, cats ferrets, llamas, and porpoises was reported in British Columbia.32 Vancouver Island was found to be heavily colonized with C. gattii, an organism formerly thought to be restricted to warm and tropical climates. The worldwide distribution of this pathogen may be changing.20 Although C. neoformans, and particularly C. gattii, cause disease in immunocompetent people, these yeasts are primarily opportunistic pathogens, and most affected humans and animals are immunocompromised, such as from human immunodeficiency virus (HIV) infection, corticosteroid treatment, chronic leukemia, lymphoma, sarcoidosis, and Cushing’s disease.1,24,25 Men are more frequently infected than women, and most cases occur in postpubertal persons.25 C. neoformans infection has also been associated with pregnancy in women.1
Although C. neoformans is an opportunistic pathogen, it can be recovered from healthy persons on the skin or in sputum, where it may be an incidental or transient colonizer.1 Most adults possess antibodies to this organism,24 and the incidence of asymptomatic infection is unknown. In a preliminary study, 3% of 61 healthy horses in New South Wales had serum antibodies to C. neoformans, suggesting previous exposure to or latent infection with cryptococci.31,33
Pathogenesis
Cryptococcal disease in healthy people or animals may result from exposure to a large dose of the yeast or to a particularly virulent strain.25 Initial exposure to the organism occurs by ingestion, contact through abraded skin or mucous membranes, or most often, inhalation of either desiccated yeast cells or (more likely) basidiospores. Basidiospores are produced in the environment by the sexual form of the C. neoformans, Filobasidiella neoformans, or from monokaryotic hyphae that develop under appropriate conditions, in the absence of mating.27 Desiccated, poorly encapsulated yeast cells usually have a diameter greater than 2 μm (too large to be easily aerosolized and readily respirable) and reduced viability, whereas basidiospores, with a diameter of 2 μm, are small enough to reach the distal airways when inspired27 and are resistant to desiccation. Mucociliary responses clear healthy airways of most inhaled infectious fungal propagules, and those that reach the alveoli are controlled by local host inflammatory responses, resulting in elimination of the fungi by alveolar macrophages, lymphocytes, neutrophils, and activated T cells. A robust cell-mediated immune response is essential to successful host defense against cryptococci, and Th1 immune responses are important for protective immunity.24
Antibodies to C. neoformans may be detected in human patients with early or focal infections.8 During active disseminated infection, antibodies are usually not detectable, perhaps as a result of forming complexes with circulating capsular cryptococcal antigens or inhibition of antibody synthesis by these antigens.8,25 In some human studies, antibody titers increase as capsular antigen titers decrease, and the patient improves clinically.33 The presence of antibody in the serum of infected persons may be an indication for an improved prognosis.8,33
Yeasts that are not destroyed by the immune inflammatory response are segregated by a chronic inflammatory process that produces granulomas. The presence of a large granuloma reflects a robust cell-mediated immune response to cryptococcal organisms.31,34 In one study, three of seven horses with C. neoformans infection had large granulomas in the dorsocaudal lung.30 The same three horses had evidence of exercise-induced pulmonary hemorrhage (EIPH), and the investigators suggested that EIPH may predispose affected lung to colonization by inhaled cryptococci.30 The dorsocaudal region of the lung receives air through the terminal ramifications of the principal bronchus and is a likely site for deposition of inhaled particles.30,35 In addition, this is a common site in which to find evidence of EIPH.
In immunocompetent persons, C. neoformans may persist indefinitely in a latent state within a granuloma.24 In theory, subsequent reactivation of latent infection may be associated with immune suppression or concurrent disease and may result in a disseminated cryptococcal infection, without evidence of recent infection.
In immunocompromised hosts, particularly those with CD4+ T-cell deficiency, cryptococcal infection disseminates within the lung, is translocated across the gastrointestinal wall after ingestion of infectious propagules or swallowing of infected nasal or pulmonary secretions, and disseminates hematogenously to other tissues, with a predilection for the leptomeninges and CNS. Dissemination from the lung may take weeks or months in human patients.8 The reason this organism favors the CNS has not been documented. Infection of the nasal cavity and the maxillary and frontal sinuses in the horse could result in meningitis and CNS invasion by direct extension through the cribriform plate.30,36 Hematogenous dispersion to the CNS has also been suggested.
Cryptococcal endometritis may be associated with chronic yeast colonization of the clitoral sinus.37
The pathogenesis of cryptococcal infections in human patients has been reviewed.21,24,25,27
Clinical Findings
A review of 40 cases of cryptococcosis in horses reported since 1902 showed that 16 horses had pneumonia or pleuritis,28,30,31,38,39 10 had upper respiratory tract infections (nasal plaques, sinus infections),28,40 10 had CNS disease,28,36,41–43 three had abdominal disease,28,30,44 two had endometritis,37,38 and one had a locally aggressive subcutaneous mass.45 One horse had both pneumonia and CNS disease,43 and another had a nasal granuloma and jejunal lesion.44
Onset of disease is often insidious, with a slow progression characterized by vague signs. Purulent nasal discharge often creamy white in color, cough, and weight loss were common signs in horses with respiratory disease. Two foals aborted by mares with cryptococcal placentitis or endometritis had cryptococcal pneumonia.46 Violent dementia, head tilt, ipsilateral weakness, unilateral blindness, ataxia, and weight loss were clinical signs of the neurologic form of cryptococcal disease. A pregnant mare with a large, abdominal cryptococcal granuloma had weight loss and poor fetal growth.30 Another horse had colic resulting from an intraluminal jejunal cryptococcal granuloma within an intussusception.28 A jejunal lesion was an incidental finding in a horse with a cryptococcal nasal granuloma.44 One horse had a large, nonhealing skin lesion that extended into the subcutaneous tissues, invading through several intercostal spaces to the pleura.45
All except two of the affected horses died or were euthanized because of extensive disease, the poor prognosis, or the expense of treatment. The survivors included one pony treated successfully with AMB for a large pulmonary cryptococcal granuloma31 and a horse with surgical resection of a jejunal intussusception involving an intraluminal cryptococcal granuloma.28
Diagnosis
Cytology
Cryptococci can be seen in clinical specimens (cerebrospinal fluid [CSF], abdominal or pleural fluid, transtracheal aspirates, BAL fluid, aspirates from infected lesions) when stained with new methylene blue, Gram stain, or Romanovsky-type stains23,30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree