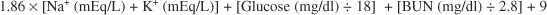29 MICROSCOPIC ASPECTS OF URINALYSIS AND DISCUSSION OF SELECTED DISEASE PROCESSES
1 What is the clinical significance of crystalluria?
The finding of crystalluria does not necessarily indicate the presence of uroliths or even a predisposition to form uroliths. A small amount of struvite or amorphous phosphate crystalluria can occur in clinically normal dogs and cats. Calcium carbonate crystalluria is a common finding in equine, goat, rabbit, and guinea pig urine samples. Detection of crystalluria may be diagnostically useful when abnormal crystal types are identified (e.g., ammonium urate, calcium oxalate monohydrate), when large aggregates of struvite or calcium oxalate crystals are found, or when crystalluria is observed in a patient that has confirmed urolithiasis. Evaluation of the type of crystals present may be useful to estimate the mineral component of the urolith(s) while awaiting results of complete urolith mineral analysis. However, the type of crystalluria present is not a definitive indicator of a urolith’s mineral content because uroliths are often heterogeneous. Additionally, sequential evaluation of crystalluria may aid in monitoring a patient’s response to therapy for urolith dissolution.
5 What is the chemical composition of the crystal in Figure 29-1, and under what circumstances do these crystals occur?
6 What is the common name and chemical composition of the crystal in Figure 29-2, and under what circumstances do these crystals occur?
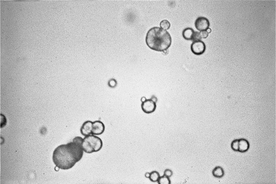
Magnesium ammonium phosphate crystals are referred to as struvite crystals or “triple phosphate” crystals (a misnomer). These are colorless and frequently form variably sized, coffin lid–shaped crystals. However, struvite crystals can have a variable appearance and may occur as three- to eight-sided prisms, needles, or flat crystals with oblique ends (see Figure 29-2). They form most often in alkaline urine. Struvite crystalluria may form in vitro in refrigerated, stored urine samples or in those that become alkaline with storage. When struvite crystals are detected in a stored urine sample, the finding should be verified by examination of a freshly obtained urine sample.
7 What is the chemical composition of the crystal in Figure 29-3, and under what circumstances do these crystals occur?
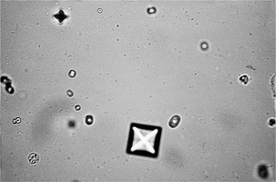
Calcium oxalate dihydrate crystals occur as colorless, variably sized octahedrons that resemble an envelope or a Maltese cross. They form most often in acidic urine. Prolonged storage, especially with refrigeration, significantly increases the potential of in vitro calcium oxalate formation. A sample that becomes acidic during storage also may lead to in vitro calcium oxalate formation. When the crystals are detected in a stored urine sample, the finding should be verified by examination of a freshly obtained urine sample. These crystals may be seen in clinically normal animals, in those with calcium oxalate urolithiasis, with hypercalciuria, (e.g., due to corticosteroids), or with hyperoxaluria, (e.g., ingestion of vegetables high in oxalates, or infrequently with ethylene glycol toxicosis). Calcium oxalate dihydrate crystals have been reported with increased frequency in cats as a complication of urine acidification to manage struvite formation.
8 What is the chemical composition of the crystal in Figure 29-4, and under what circumstances do these crystals occur?
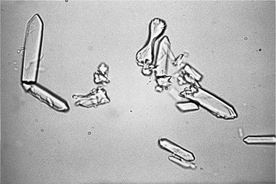
Calcium oxalate monohydrate crystals are colorless and variably sized. These crystals typically are flat with pointed ends and appear similar to hemp seeds or picket fence boards (see Figure 29-4). Less often, they may occur as spindle- or dumbbell-shaped crystals. Although either calcium oxalate monohydrate or dihydrate crystals can be seen with acute ethylene glycol toxicity, the monohydrate form is more diagnostic of intoxication, since this form is usually only seen during acute ethylene glycol toxicity and is rarely found in clinically normal animals. Formation of crystals is time dependent and occurs only during the early phase of intoxication. Crystalluria may be observed within 3 hours of ingestion in cats and within 6 hours in dogs and may last up to 18 hours after ingestion.
9 What serum biochemical information can also be used to support the diagnosis of ethylene glycol intoxication?
10 What is the chemical composition of the crystal Figure 29-5, and under what circumstances do these crystals occur?
11 What is the chemical composition of the crystal in Figure 29-6, and under what circumstances do these crystals occur?
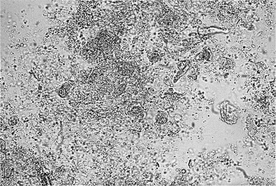
Amorphous phosphates and urates have a similar shape and may occur as amorphous debris or small spheroids. Amorphous phosphates are distinguished from amorphous urates in two ways: phosphates are colorless and precipitate in alkaline urine, whereas urates are yellow-brown to black and precipitate in acidic urine. Amorphous phosphates are a common finding in alkaline urine of clinically normal animals. They are of no known diagnostic significance. However, amorphous urates are uncommon in clinically normal dogs and cats. They may be seen in animals with portovascular malformation, severe hepatic disease, or ammonium urate urolithiasis. Amorphous urates are often seen in Dalmatians and English bulldogs and may represent a predisposition to urate urolithiasis in these breeds. Dalmatians have defective purine metabolism and do not convert uric acid to allantoin. (Most other breeds convert uric acid to allantoin, which is water soluble and excreted in urine.) Additionally, Dalmatians may also have decreased tubular resorption of uric acid.
12 What is the chemical composition of the crystal in Figure 29-7, and under what circumstances do these crystals occur?
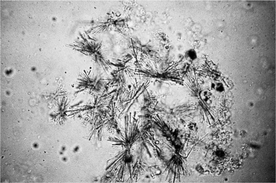
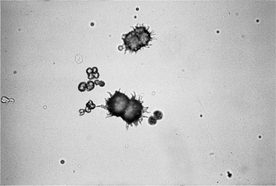
Ammonium urate crystals may also be referred to as ammonium biurate crystals. They are golden to brown and spherical with irregular protrusions, giving the appearance of a thorn apple or sarcoptic mange mites (see Figure 29-7). In cats, they may occur as smooth aggregates of spheroids. Ammonium urate crystals are seen in animals with portovascular malformation and with severe hepatic disease and infrequently in clinically normal Dalmatians and English bulldogs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree