27 James M. Fingeroth and William B. Thomas Even with an accurate diagnosis and specific therapy such as surgery, if subsequent nursing care is inadequate, the patient with intervertebral disc herniation (IVDH)-induced paralysis will not recover optimally and may suffer unnecessary discomfort or pain or even fatal complications. This chapter reviews basic principles of nursing care in the recumbent patient, and applies equally to those being managed medically and those recovering from surgery. A critical aspect of caring for a paralyzed patient is early assessment of the animal’s ability, or lack thereof, to void urine voluntarily. Urine retention is too often overlooked during initial evaluation. This can lead to unnecessary discomfort for the animal and predispose to secondary problems such as urinary tract infection (UTI) and detrusor atony. The failure to identify and initiate early intervention for this problem probably stems from several factors. These include being overly focused on limb dysfunction and forgetting to consider the likelihood of concomitant urinary dysfunction, and misinterpreting overflow incontinence as voluntary micturition. An early part of client communication/education should also touch on the need for bladder management, as this may be something the clients have to learn and assume control over once a pet is discharged from the hospital. As a rule of thumb, most dogs and cats recover voluntary control over micturition concomitant with recovery of voluntary/purposeful appendicular motor function (even if yet nonambulatory). Micturition is the process of storing and periodically voiding urine. This involves a complex series of neural pathways that controls the urinary bladder and urethra. The primary control center for micturition is located in the pons. Other brain regions, including the forebrain and cerebellum, are important for voluntary storage of urine and initiation of voiding. Axons projecting from the micturition center travel caudally in the spinal cord to the lumbar and sacral segments that innervate the bladder and urethra. Sympathetic neurons in the lumbar spinal cord segments (L1–L4 in the dog, L2–L5 in the cat) provide axons to the hypogastric nerves that innervate the detrusor muscle in the bladder (β-adrenergic receptors) and the smooth muscle of the urethra (α-adrenergic receptors). These act to inhibit detrusor muscle contraction and increase urethral tone during the storage phase of micturition. Parasympathetic neurons in the sacral spinal cord segments innervate the detrusor muscle via the pelvic nerves and act to contract the detrusor muscle during voiding. General somatic efferent neurons in the sacral segments innervate the skeletal muscle of the urethra via the pudendal nerves. This provides voluntary contraction of the urethra that is important in storage of urine. During storage, sympathetic tone predominates to relax the detrusor muscle to accommodate filling with urine against a closed outlet provided by a contracted urethra. As the bladder becomes full, stretch receptors in the bladder are activated and project sensory information along the spinal cord to the brain so that the animal is aware of bladder distension. Conscious voiding occurs by voluntary release of inhibition of the micturition center in the pons. Parasympathetic tone predominates during voiding. Impulses travel along the spinal cord to the lumbar and sacral segments to relax the urethra in coordination with contraction of the detrusor muscle [1, 2]. In most cases of intervertebral disc herniation, the spinal cord lesion is cranial to the sacral spinal cord segments. If the lesion is severe enough, it damages the ascending sensory pathways and descending motor pathways responsible for micturition and prevents voluntary voiding. Detrusor and urethral tones are increased because of loss of inhibition from the brain. The bladder becomes full and feels firm and turgid, and there is substantial resistance to manual evacuation of the bladder. There may be inconsistent leakage of urine from an overly full bladder (overflow incontinence). This syndrome is referred to as an upper motor neuron (UMN) bladder. Over a period of days to weeks, some patients will develop a variable degree of reflex voiding. Bladder distension activates neurons in the sacral segments that serve to contract the detrusor muscle and relax the urethra. However, this voiding is involuntary and occurs only to the point where detrusor stretching is sufficiently relieved. At that point, the situation returns to status quo ante, where the bladder still retains urine. In some cases, this reflex is stimulated by abdominal pressure such as picking up the patient. It is critical to not mistake this reflex and incomplete voiding as evidence of voluntary micturition. Much less common in patients with disc herniation is a lesion affecting the sacral spinal cord segments or nerve roots. This results in loss of voluntary micturition accompanied by decreased tone in the detrusor muscle and urethra. The bladder feels flaccid and is easily expressed. Overflow incontinence is common when the bladder is distended and the patient often leaks urine spontaneously or in response to abdominal pressure. This syndrome is called a lower motor neuron (LMN) bladder [1, 2]. In some cases, smooth muscle sphincter tone (innervated by the hypogastric nerve) may be preserved and become static such that outflow resistance is preserved [1]. In these patients, it may be difficult to manually express the bladder. Therefore, the assessment of urethral tone alone is not always an accurate way to localize spinal cord lesions. These patients often have decreased anal tone and absent perineal reflexes and may have loss of sensation in the perineum and tail. These findings are more reliable in localizing the lesion to the sacral segments and nerve roots, and underscore the need to assess anal tone and perineal reflexes when evaluating paralyzed patients. Animals with severe paresis or paralysis should be suspected of having micturition compromise as well. Urine retention increases the risk of UTI, and overdistension of the bladder can damage the detrusor muscle resulting in persistent atony [1, 3]. Patients may leak urine due to decreased urethral tone (LMN bladder) or overflow incontinence (UMN) or incompletely void urine due to reflex voiding. Therefore, finding urine in the patient’s cage or bedding is not a reliable indicator that the animal can urinate voluntarily. If possible, the patient is taken outside and given adequate time to urinate voluntarily. Even if the patient voids, the bladder is palpated to assess residual volume. Normal residual volume after voiding is 0.2–0.4 ml/kg (usually <10 ml total) [1]. It may be difficult to palpate the bladder in obese patients or painful patients with increased abdominal muscle tone. In those cases, ultrasound is useful to assess bladder size. In general, the bladder should be assessed every 6 h, but this is adjusted as necessary in individual patients. For example, patients receiving intravenous fluid therapy or corticosteroids often have increased urine volume and require more frequent assessment. In patients that do not urinate voluntarily or have excess residual volume, the first step is to attempt manual bladder expression. This is performed outside, over absorbent bedding, or over a drain. Expression can be done with the patient in lateral recumbency or supported in a standing position, whichever is more comfortable. Place a hand on each side of the abdomen, just caudal to the last rib. Gently palpate the abdomen by advancing your hands medially and caudally until you can feel the bladder. Then apply slow, steady pressure with the flat portions of the hands and fingers to completely empty the bladder. If the patient starts to tense the abdominal muscles, release pressure until the patient relaxes and then start again. Never try to “overpower” the patient. If the bladder cannot be comfortably expressed, the next step is catheterization. Sedation is often helpful for patient comfort. For male patients, a sterile, soft urethral or feeding tube is premeasured from the tip of the penis to 2–4 cm cranial to the pubis to ensure appropriate length to reach the urinary bladder. The patient is physically restrained in lateral recumbency with the aid of an assistant. The penis is exposed and the urethral opening cleansed with antiseptic solution. Wearing sterile gloves, the lubricated sterile catheter is passed into the urethral opening and into the urinary bladder. The bladder is emptied with a syringe until urine can no longer be obtained [4]. Female patients are positioned in lateral or sternal recumbency, and the perivulvar region is clipped, cleaned, and prepared with antiseptic. A stylet is often useful. A light and a sterile vaginascope or an otoscope with a sterile speculum is helpful in visualizing the urethral papilla. The catheter is inserted through the papilla and into the bladder to a premeasured length in a sterile fashion. In females or other patients where catheterization is expected to be difficult or uncomfortable for the patient, it is often beneficial to place an indwelling silicone Foley urinary catheter. In patients undergoing diagnostic imaging and/or surgery, this can be accomplished under general anesthesia at the time of imaging or surgery. Once urine flows from the catheter, the balloon at the tip of the catheter is inflated with sterile saline and the catheter connected to a sterile, closed urine collection system. Urine is aseptically drained from the collection system two to four times daily and urine volume recorded. The exposed portion of the catheter is cleaned daily with antiseptic solution. Once the patient has recovered strong motor function in the pelvic limbs, or one anticipates being able to accomplish manual bladder emptying, the urinary catheter may be removed and the patient observed for voluntary urination. Although urethral catheterization carries a risk of introducing bacteria, one study did not find a statistically significant difference in the rate of UTI in patients managed with intermittent catheterization or indwelling catheterization, compared to manual expression [4]. A longer duration of catheterization is associated with a progressive increase in the risk for UTI, so catheterization is stopped as soon as the patient is able to urinate voluntarily [2, 4]. Empiric antibiotic therapy for prophylaxis during the period of indwelling catheterization is generally contraindicated because this increases the risk of UTI and antibiotic resistance [5]. Pharmacologic treatment is most effective when started early in the course of treatment and used for short periods of time. Drug therapy is helpful in altering detrusor and urethral tone, but no drug will restore voluntary urination in a patient with spinal cord disease. Drug therapy is only one component of management and not a substitute for other therapies such as manual bladder expression or catheterization. Commonly used drugs are described in Table 27.1. Table 27.1 Drugs used to manage urine retention Pharmacologic manipulation of outlet resistance may be directed at smooth or skeletal muscle components of the urethra. Drugs to decrease urethral tone are most commonly used in patients with UMN bladders to facilitate manual bladder expression. Phenoxybenzamine is a nonselective α-adrenergic antagonist that decreases urethral resistance. It is administered orally at dosage of 5–20 mg every 12 or 24 h in dogs or 2.5–5 mg every 12 or 24 h in cats. Side effects can include gastrointestinal upset and hypotension. It often takes several days of therapy for clinical effects to become apparent. Phenoxybenzamine is considered obsolete for the treatment of functional urethral obstruction in human patients, following the advent of selective α-1 antagonists, and concerns regarding the potential carcinogenicity of phenoxybenzamine. This has limited the availability of the drug in certain regions and resulted in an increase in price [6]. Prazosin is a readily available and inexpensive selective α-1 antagonist. It is administered orally at 1 mg per 15 kg every 8 or 12 h in dogs and at 0.25 mg per cat every 12 or 24 h [1]. Side effects are similar to those of phenoxybenzamine, especially hypotension. To decrease the risk of hypotension, half the calculated dose is often administered for the first few days of treatment as the patient is monitored for signs of hypotension, such as lethargy or syncope. Skeletal muscle relaxants provide additional relaxation of the urethra in patients with UMN bladders, especially in male dogs and in cats, which have greater proportions of skeletal muscle in the urethra. In dogs, diazepam is administered orally at 2–10 mg per dog every 8 h. The dose for cats is 1–2.5 mg orally every 8 h [1]. In patients that are managed with manual bladder expression, the dose is administered 30– 60 min prior to attempting bladder expression. The most common side effect is mild sedation. Some cats have developed hepatic failure after receiving oral diazepam for several days. Clinical signs include anorexia, lethargy, ataxia, and jaundice [7]. Cats that receive diazepam should have baseline liver function tests, and the drug should be discontinued if clinical signs of toxicity develop. Dantrolene is a direct-acting skeletal muscle relaxant that has been used to reduce urethral pressure. It is administered orally at 1–5 mg/kg every 8 h (dogs) or 2 mg/kg every 8 h in cats [1]. Potential adverse effects include sedation, hypotension, and gastrointestinal upset [1]. UTI is a common complication of thoracolumbar disc-induced spinal cord disease, occurring in 27–42% of patients [3–5, 8]. Risk factors for the development of UTI include loss of ambulation, inability to voluntarily urinate, duration of inability to urinate, and body temperature less than 35 °C during anesthesia [4, 5, 8]. Perioperative administration of cefazolin decreased the risk of UTI in one study [8]. Escherichia coli and Enterococcus sp. are the most common isolates. Others include Klebsiella pneumoniae, Staphylococcus intermedius, Streptococcus sp., Enterobacter sp., Acinetobacter sp., and Proteus mirabilis. Many UTIs are occult, with no clinical signs and no hematuria or pyuria detected on urinalysis [4, 8]. Given the high incidence of UTI and the frequency of occult infections, one of the authors (WT) routinely collects urine for culture at the time of surgery and again once the patient regains voluntary urination and any catheterization has ceased. Any confirmed UTI is treated with a 10-day course of antibiotic chosen on the basis of susceptibility testing. Several days after antibiotic therapy is discontinued, another urine sample is submitted for culture to ensure the infection is eradicated. Although both urinary continence and fecal continence are affected with the disturbance of sphincter function of both UMN and LMN, there is less concern generally with the fecal continence in dogs and cats. Animals with lesions cranial to S2 may well have some degree of constipation, but we rarely recognize overt signs of discomfort in patients with fecal retention, and the reflexive emptying of stool appears to occur with much less effort than with urine emptying. Quite often, one will observe defecation simultaneously with manual bladder expression, presumably because the stimulation provided during manual expression initiates a reflexive impulse in the pudendal nerves that subserve both bladder/urethral and anal sphincter relaxations. The propulsive peristaltic nature of ingesta and fecal transit through the gastrointestinal tract also probably influences the movement of stool through the anal sphincter despite hypertonicity/hyperreflexia. As with LMN urine incontinence described earlier, the key nursing care element is prevention of fecal soiling and subsequent dermatitis, as the patient may be unaware of (and unable to move away from, due to paralysis) any bowel movement. With both LMN fecal and urinary incontinence, the patient is likely to become soiled with excretions. Nursing care thus demands minimizing the dermatologic effects from such soiling. Fecal soiling can be combated by frequent examination and cleansing, shaving the perineum, and wrapping the tail. Appropriate salves can also be employed to soothe irritated skin. Patients with acute paralysis associated with IVDH may have anxiety and discomfort. These may adversely affect intake of food and water. A short period of hyporexia or anorexia is probably not harmful to most patients, but dehydration is anathema for patients with spinal cord injury, as this may cause a reduction in spinal cord blood flow in a situation where spinal cord circulation in the injured segments may already be tenuous. It is therefore always a consideration as part of the early and ongoing nursing care and management of patients with IVDH and neurologic deficits to provide intravenous fluid support. Adequate nutrition is important to slow catabolism and provide precursors for optimal immune function, tissue repair, and drug metabolism. The resting energy requirement (RER) is estimated by the following formulas:
Medical Management and Nursing Care for the Paralyzed Patient
Bladder management
Control of micturition
Upper motor neuron bladder
Lower motor neuron balder
Management of urine retention
Pharmacologic treatment of urine retention
Drug
Action
Dose
Possible adverse effects
Diazepam
Centrally acting skeletal muscle relaxation
Dog: 2–10 mg q8 h, PO
Cat: 1–2.5 mg/cat q8 h, PO
Sedation
Paradoxic excitement
Hepatic necrosis (cats)
Dantrolene
Direct-acting skeletal muscle relaxation
Dog: 1–5 mg/kg q8 h, PO
Cat: 2 mg/kg q8 h, PO
Sedation
GI upset
Phenoxybenzamine
Smooth muscle relaxation
Dog: 5–20 mg q12–24 h, PO
Cat: 2.5–5 mg q12–42 h, PO
Hypotension
Tachycardia
GI upset
Prazosin
Smooth muscle relaxation
Dog: 1 mg per 15 kg q8–12 h, PO
Cat: 0.25 q12–24 h, PO
Hypotension
Mild sedation
UTI
Defecation
Hydration and nutrition
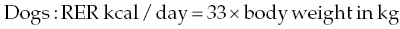
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


