CHAPTER 2 Mechanisms of Infectious Disease
NORMAL FLORA
Dermal and mucosal surfaces provide a life-preserving protective barrier composed of physical, chemical, and microbial defenses.1 Normal flora is an essential component of this protective barrier against pathogens yet paradoxically provides a source for opportunistic invasion. Commensal bacteria are those living on or within a host in a way such that both derive mutual benefit and in which interruption of this association results in abnormal host development or overt disease.1 A pathogen is any disease-producing organism; thus a commensal is potentially pathogenic. Colonization is infection without disease; colonization of skin, gastrointestinal, respiratory, and urogenital tracts occurs early in life and persists during the healthy lifetime of the host.
Skin Flora
The combination of normal flora and mucosal immunity provides an effective barrier against infectious colonization of nondisrupted skin surfaces. Specific bacteria are stratified by site, and certain bacteria multiply and colonize depending on associated adnexa.2 Most bacteria and fungi on the surface of the skin are not associated with disease; however, yeast and bacteria associated with hair follicles are more likely to be related to a disease process.3 Even though horses inhabit an environment heavily contaminated with fecal flora, normal dermal flora in the horse is surprisingly devoid of members of the Enterobacteriaceae.4 Normal inhabitants include mixed populations of bacteria of species of Acinetobacter, Aerococcus, Aeromonas, Bacillus, Corynebacterium, Flavobacterium, Micrococcus, Nocardia, coagulase negative Staphylococcus, Staphylococcus aureus, Streptomyces, and nonhemolytic Streptococcus generae.2 Certain Staphylococcus spp. have been associated with skin disease in the horse, and these include S. aureus, S. intermedius, and S. hyicus, whereas species such as S. xylosus and S. sciuri were more associated with normal skin.
More than 30 species of fungi can inhabit the skin and Alternaria, Aspergillus, Candida, Fusarium, Rhizopus, and Trichophyton spp. are commonly present.2 Until recently the presence of Malassezia yeast species has been considered pathogenic. Recent fungal culturing of the skin of normal, healthy horses has confirmed colonization by a species of Malassezia yeast that is novel to the horse (tentatively named M. equi). Colonized sites include groin, axilla, and perineal regions.5
Oral, Pharyngeal, and Respiratory Flora
Oral and pharyngeal mucosal flora is associated with both health and disease of the upper and lower respiratory system. The oral and pharyngeal mucosa is richly populated with many bacteria, including obligate aerobes, anaerobes, and facultative anaerobes.6 Gram positive and negative anaerobes are the predominant flora in the mouth and pharyngeal tonsils of the normal horse, with B. fragilis and Bacteroides spp. most commonly found. Genera consisting of Fusobacterium spp., Eubacterium spp., Clostridium spp., Veillonella spp., and Megasphaera spp. are also cultured. Aerobic and facultative anaerobic populations mostly comprise S. zooepidemicus, Pasteurella spp., E. coli, Actinomyces spp., and Streptococcus spp. Because these same genera are also consistently found in horses with lower respiratory infections, opportunistic colonization by pharyngeal flora is the likely mechanism of disease.6 Contamination of the trachea of the horse is a frequent occurrence, as evidenced by the fact that trans-tracheal aspiration (TTA) yields positive bacterial cultures in approximately 30% of both normal adult horses and foals.7 As with skin flora, normal horses have multiple fungal species inhabiting conjunctival, nasal, and oral mucosa.8 Stabling increases the frequency of ocular fungi in normal horses.8
Intestinal Flora
In animal models and chronic human conditions, normal flora is considered important for intestinal maturity and containment of disease. Changes in cecal weight, villus:crypt ratio, and volatile fatty acid production and the development of gut IgA responses are all affected by suboptimal cecal colonization in germ-free animals.9 A relationship between severity of mucosal disease and normal flora has also been demonstrated in models of inflammatory bowel disease of humans.10
Bacteria are present in all parts of the intestinal tract of the horse, and the microbial fauna increases in complexity and density aborally.11 The stomach of the horse is not a sterile environment. A dense population of gram positive bacterial rods, primarily composed of Lactobacillus spp., colonizes the nonsquamous portion of the equine stomach. Substantial colonization of the duodenum is present with a large population of proteolytic bacteria, and this colonization increases by tenfold in the ileum.12
Microbial degradation and fermentation of plant material in the large intestine are important components of nutrient acquisition in the equid. The consumption of cellulose and starch results in the production of volatile fatty acids (VFAs).13 The major cellulolytic bacterial strains in the horse produce arrays of fermentation products that differ from those of cattle.14 The common bovine rumen bacteria Ruminococcus flavefaciens is one of the most predominant cellulolytic bacteria of the equine cecum based on standard microbiologic techniques.15 Genetic techniques also demonstrate that the predominant flora are the low GC-content bacteria, which include Cytophaga–Flexibacter–Bacteroides and Clostridium bacteria; the actual species are completely novel.15 Standard microbiologic techniques specifically demonstrate Enterobacteriaceae, Butyrivibrio spp., Streptococci spp., Bacteroides spp., Lactobacilli spp., Selenomonas spp., Eubacterium spp., Propionibacterium spp., and Staphylococcus spp. in residence.16 In addition, there are completely different compositions of bacteria between the differing segments of the colon, especially between the ascending colon and cecum, indicating highly specialized digestive functions associated within the large intestine itself.15 Yeasts and fungi of the order Mucorales have been identified in the cecum of normal horses and are capable of digesting cellulose and starch.17
Routine surveillance demonstrates a relative lack of intestinal pathogens in the flora of normal horses. In the largest study to date, fecal shedding of Salmonella enteriditis as detected by fecal culture in normal horses from farms without evidence of salmonellosis was 0.8% in resident horses.18 Molecular diagnostics, once hoped to provide a tool for understanding the incidence of Salmonella spp. in the clinically normal horse, has provided inconsistent information, and polymerase chain reaction (PCR) is most useful for identification of subclinical shedders and environmental contamination during an outbreak.19,20 On the basis of limited investigations, the carriage rates of C. difficile in normal horses and foals appear to be low (<1.5%).21
Intestinal flora in the horse is an important source for extraintestinal pathogens. In studies examining the carriage rate of Rhodococcus equi, all horses cultured carried the bacteria regardless of age.22,23 Furthermore, if the farm had endemic R. equi and respiratory isolates contain the 90 kDa plasmid, which has been associated with disease, fecal isolates also contained this plasmid.
Urogenital Flora
By far, most of the work that characterizes equine normal flora has focused on urogenital flora to address infertility and fetal loss. Although vaginal and vestibular mucosa of mares should be colonized with normal mucosal flora, the uterus is considered sterile. However, typical culturing techniques result in frequent isolation of what could be considered pathogens, and cytology and bacterial counts are essential supplemental tests for detecting true uterine infection. Counts less than 10 colony-forming units and lack of inflammatory cells indicate uterine or technical contamination.24
Many bacteria inhabit the external genitalia of stallions, including bacteria considered to be associated with metritis in mares. The predominant aerobe isolated is coagulase-negative Staphylococcus spp., followed by Corynebacterium spp., α-hemolytic Streptococcus spp., and Lactobacillus spp. Pathogens such as β-hemolytic Streptococcus spp., Pseudomonas aeruginosa, and Klebsiella spp. can be frequently found in servicing stallions.25,26 Pregnancy rates appear to be the same in mares bred to P. aeruginosa semen–infected stallions.27
POPULATION BIOLOGY OF BACTERIAL INFECTIONS
Inoculum Size
Little dose-response work has been published that demonstrates a clear relationship between frequency or severity of disease and dose for many equine bacterial pathogens. In general, for systemic infections, inoculum size has been shown to be the most important determinant of disease in hematogenously disseminated infections such as meningitis and, in rodent models, osteomyelitis.28 The equine literature emphasizes animal and environmental risk factors29 as important determinants in survival for conditions such as neonatal osteomyelitis, but these factors ultimately control the degree of bacterial challenge at birth. For skin infections, colonization of experimentally induced wounds with Staphylococcus spp. is historically dependent on inoculum size. However, attainment of antibiotic resistance and new virulence factors are predominant factors in the development of human S. aureus colonization and disease.30,31 The occurrence of diarrhea is dose dependent in salmonellosis in calves,32 but little is known regarding challenge dose in the foal or the adult horse. Very small numbers of Streptococcus equi subsp. equi inoculated intranasally result in rapid colonization of the equine lingual and nasopharyngeal tonsils within hours.33 Minimal inoculum size is required for certain pathogens that form toxins, such as E. coli O157H7and Clostridium spp. in humans.34
Virulence
Virulence is the relative ability of an organism to cause disease.1 Virulence of an organism is frequently tested by inoculation of different strains or genetically modified organisms of a pathogen into groups of a rodent species (or cell culture), and lethality or invasiveness is evaluated. When this particular methodology is used, the severity of many diseases frequently is found to be strain dependent and virulence is commonly associated with certain phenotypic characteristics of a particular strain.
Antibiotic Resistance
Widespread use of antibiotics in both animal and human infection occurred after World War II.35,36 Within 30 years resistance of gram positive organisms was already occurring in human pneumococcal infections.37 From the 1960s to the 1980s, staphylococcal resistance progressed from initial methicillin-resistant organisms to vancomycin-resistant organisms.36 Specific types and mechanisms of bacterial resistance are discussed in association with particular diseases. Briefly, there is intermediate resistance that occurs in geographically defined isolates in a step-wise fashion resulting from genetic changes under antibiotic pressure.37,38 Thus proper dose and length of antibiotic exposure are important in preventing the development of these isolates.37,38 In high-grade and multidrug-resistant (MDR) forms of antibiotic resistance, there is clonal expansion of small numbers of isolates.39 In addition to selective antibiotic pressure, individual carrier animals and people are important for development and maintenance of high-grade and MDR resistance. Virulence and antibiotic resistance are not synonymous; commensal or relatively noninvasive pathogens have higher rates of resistance,40 whereas more highly pathogenic or invasive organisms have less resistance. Because commensal organisms are ubiquitous, with a higher likelihood of contact with bacteria that have multiresistance genes, commensal organisms become a reservoir for resistance genes.40
DEVELOPMENT OF DISEASE AND THE ROLE OF NORMAL FLORA
Disruption of Normal Flora
The pathophysiology of certain types of equine colitis and pleuropneumonia is consistent with either disruption of normal flora and invasion by a pathogen or the conversion of a common commensal organism into a pathogen. Development of colitis in the horse has been associated with feed change, antibiotics, surgery, nonsteroidal inflammatory drugs, and transport, all events that disrupt flora.41,42 Rapid change from a roughage diet to concentrate results in increased anaerobes, decreased cellulolytic bacteria, decreased cecal protozoa diversity, and decreased pH in the equine cecum.41 Isolation of Clostridium difficile is more likely from horses treated with antibiotics, and clinical disease has been associated with ampicillin, erythromycin, penicillin, and potentiated sulfonamide administration in adult horses.43,44 In ponies infected with Salmonella spp., transport and surgery reactivated infection and diarrhea, and antibiotics (oxytetracycline) prolonged shedding but did not induce recrudescence.42 In a case control study, use of potentiated sulfonamides was not significantly associated with the development of diarrhea in hospitalized horses; however, overall antibiotic use was highly associated with the occurrence of diarrhea.45
There are several mechanisms by which antibiotics disrupt normal gastrointestinal flora and function.46 The most important include (1) disruption of carbohydrate metabolism, (2) decreased metabolism of bile acids, (3) direct effects on intestinal motility, and (4) shortening of intestinal villi. Changes in carbohydrate metabolism are a large intestinal event secondary to reduced microbial reduction of carbohydrates to short-chain fatty acids (SCFAs). Because SCFA metabolism and absorption result in fluid and electrolyte absorption, a sudden decrease in SCFA leads to osmotic diarrhea with an intraluminal accumulation of organic acids, cations, and carbohydrates. In human studies, reduced SCFAs have been demonstrated with many antibiotics, including ampicillin, metronidazole, and erythromycin.46 Bile acids, reduced in the colon by dehydroxylating bacteria, are potent colonic secretogues. Increases in fecal bile acids have been demonstrated in humans with the use of ampicillin and clindamycin.46 Erythromycin and amoxicillin directly affect colonic motility.46 Erythromycin is a motilin receptor agonist resting in contraction of antral and duodenal smooth muscles.47 In the horse erythromycin results in a dose-dependent increase in ileocecal emptying.48 Motility enhancing effects have also been observed in human patients treated with amoxicillin.46
Disease Caused by Colonization of Commensal Flora
Occurrence of infectious lower respiratory disease in adult horse is an example of how contamination of a normally sterile site with several commensal bacteria results in disease. Changes in upper respiratory mucosal flora and transportation are two important elements that contribute to the development of pleuropneumonia in the horses. The tonsillar mucosa of the oropharynx is heavily colonized with S. equi subsp. zooepidemicus, and necrosis of this tissue occurring during viral infection is associated with spread to the lower respiratory tract.6 Transport of horses (especially for distances >500 miles) is a primary risk factor for pleuropneumonia as demonstrated in a large retrospective study.49 Elevation of the head for an extended period of time is likely a contributing factor. Horses normally feed from the ground for most of the day, and this posture promotes effective tracheal clearance of inhaled debris and particulate matter. Under experimental conditions elevation of the head for prolonged periods of time results in an increase in the variety and multiplication of oral/pharyngeal commensal bacteria within the trachea. Pasteurella, Actinobacillus, and Streptococcus spp. are the most frequent and prolific colonizers of the trachea after prolonged head elevation.50,51 In addition to prolonged head elevation imposed by extended transport periods, there is decreased phagocytosis of equine peripheral neutrophils in these horses.51 As a result, common commensal bacteria of the nasal and oropharyngeal mucosa become opportunistic pathogens.
Nosocomial Infections
Nosocomial infection (health care–associated infections) is defined by the Centers for Disease Control and Prevention (CDC) as a localized/systemic condition resulting from an adverse reaction to the presence of an infectious agent or its toxin. There must be no evidence that the infection was present or incubating at the time of hospital admission.52 Nosocomial infections are becoming a major problem for large animal veterinary teaching and private referral hospitals. Infections with Serratia marcescens, Acinetobacter baumannii, S. aureus, methicillin-resistant Staphylococcus spp., Enterococcus spp., and various Salmonella enteritidis serovars have all been reported in association with nosocomial infection in equine patients.53,54 Surgical incision infection, joint sepsis, catheter phlebitis, wounds, and diarrhea represent the common clinical syndromes reported in horses.53-55 When nosocomial infection involves the acquisition of isolates from the hospital environment, these isolates are more difficult to treat because they frequently undergo high-level antibiotic pressure and attain multiresistance. Nosocomially transmitted salmonellosis in equine hospital wards is increasingly reported, with Salmonella enteritidis serotypes Krefeld, Saint Paul, DT104, and Anatum all demonstrating attainment of MDR over the course of the outbreak.56,57 Only one study of a nosocomially transmitted Salmonella enteritidis (serotype Heidelberg) did not demonstrate significant acquisition of multidrug resistance over time.58
PATHOGENESIS OF BACTERIAL INFECTIONS
The ability of bacteria to gain entry and cause disease results from a combination of factors possessed by the agent itself, environmental conditions, and status of host defenses. Bacteria either gain entry through a body surface by direct inoculation or colonize and damage a dermal or mucosal barrier to cause disease. Environmental or risk factors specific for individual diseases increase the probability of successful penetration or colonization and are discussed for specific diseases in various chapters. Innate immunity and specific immunity, which alter host susceptibility to disease, are also discussed elsewhere in this chapter. General mechanisms that are specific to bacteria and enhance disease are virulence factors that enhance the entry, spread, and damage to host tissues (Table 2-1). Virulence factors may either allow bacteria an advantage to gain entry and disseminate or directly cause damage to the host once entry has been gained. Major virulence factors are listed for specific equine pathogens.
TABLE 2-1 General Mechanisms of Bacterial Pathogenesis
| Action | Mechanism | Examples |
|---|---|---|
| Entry of bacteria | ||
| Enhancement of spread | ||
| Damage to host membranes | Toxins |
FACTORS THAT ENHANCE ENTRY OF BACTERIA
Adhesion, Entry, and Secretion
Protein secretion systems (PSSs) are a structurally diverse complex of essential virulence factors for bacteria that allow specialized interactions between cells.59 Classifications of associated pili are separated on the basis of their biogenesis by a particular PSS and their resulting final structure, which accounts for their wide diversity. These main systems function to translocate various sized molecules and are important in the formation of adhesins upon attachment to host cells. Fibrillar adhesins (FAs) and nonfibrillar adhesins (NFAs) are the most important PSS subgroup not used for bacterial conjugation. They specifically target host cells and biofilms for enhancement of colonization and invasion.
Fibrillar Adhesions
There are multiple types of FAs in both gram positive and gram negative bacteria, with gram negative the most well characterized (Table 2-2).60–62 Pili or fimbriae are rod-shaped structures composed of an orderly array of a single protein usually arranged in a helical fashion to form a cylinder. The tip of the fimbria mediates attachment to carbohydrate moieties on cell surfaces and is integral to bacterial invasion and colonization. Bacteria can also contain multiple types of pili. Both the bacterial pili themselves and the cellular pathways they use for secretion and formation of pili are targets for pharmacologic intervention, and there are multiple subclasses depending on their configuration.60
TABLE 2-2 Major Types of Bacterial Adhesins
| Adhesin | Definition | Example |
|---|---|---|
| Type 1 fimbriae | Cell surface structure primarily on gram (–) bacteria that bind to the terminal mannose of glycoproteins on cells | Escherichia coli |
| Type 4 pili | Cell surface structure primarily on gram (–) bacteria that function in adhesion, twitching, and DNA uptake, which bind on CD46 and other glycolipids | Pseudomonas aeruginosa |
| Curli fimbriae | Coiled aggregative fimbrial structures that bind to fibronectin, laminin, and plasminogen and function in adhesion, aggregation, and biofilm formation | |
| Fibrils and flexible rods | Short, thin rodlike adhesins that bind to fibronectin for adhesion to host tissues | Streptococcus species |
| (Lipo) Teichoic acid | Part of the peptidoglycan layer of cell walls | Gram positive bacteria |
| Biofilm | Exopolysaccharide produced by bacteria that allows matrix formation of embedded material | Gram positive and negative bacteria |
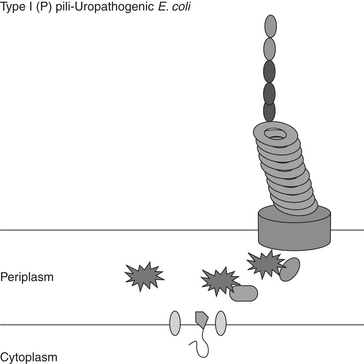
Type I fimbriae (Figure 2-1) are distributed in the periplasmic space and translocated to the cell surface by a chaperone-usher (C-U) pathway.62–65 These pili are made up of multiple major pilin subunits compiling a rigid shaft with minor pili proteins composing the flexible tip. These require a C-U secretion system for biogenesis. The C-U prevents the pili from achieving their final figuration while in the periplasmic space located between the inner and outer membrane before assuming their position on the outer membrane. Although once considered Type I, the formation of the pili associated with enterotoxigenic E. coli (ETEC) is now considered an alternate C-U pathway, which functions to confer species-specific binding to intestinal epithelium and mediate agglutination of erythrocytes. In gastrointestinal and urinary tract infection, fimbriae are likely one of the most important virulent factors for successful invasion.61 Although the importance of E. coli adhesive fimbriae is questionable in equine disease and specific receptors for these fimbriae have not been identified, their action provides the general framework of bacterial-host cell interaction. Once receptor-mediated attachment occurs, intracellular calcium increases in the host cell.61 Proteins and protein kinases involved in the breakdown of actin are activated, resulting in the disruption of microvilli. There is a change in the cytoskeleton of the cells and permeability to ions and water. Ions are secreted, resulting in the classic secretory diarrhea.
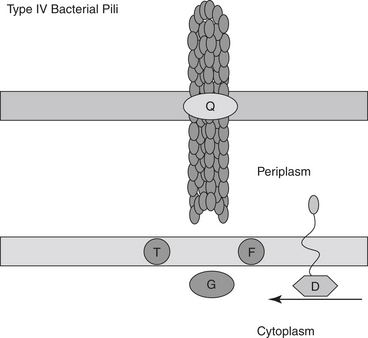
Type IV pili (Figure 2-2) are located at the pole of the cell and assembled via the Type II secretion system that is called the general assembly pathway.62,63 These pili are flexible fibers of variable length that can aggregate, are important in the formation of microcolonies, and are responsible for the twitching motion of bacteria. The proteins are secreted into the periplasmic space but remain anchored to the inner membrane. The protein is then assembled and passed through a pore formed in the outer membrane. Curli fimbriae are solid surface structures that use the extracellular nucleation-precipitation pathway.63 This pilus is secreted as a soluble protein extracellularly with a second protein, the nucleator, for stability. These have been identified as important for binding to extracellular matrix proteins and biofilm formation.
Although discussed in greater detail later, the antiphagocytic M-protein of Streptococcus spp. is actually a fibrillar protein and also assists, but is not essential for, adhesion. In anaerobic bacteria, antibodies to flagella proteins can block Clostridium difficile adhesion.66 These proteins are thought to mediate intestinal adherence and colonization.66
Afimbrial Adhesins
Afimbrial adhesins are cell proteins that enhance the binding of bacteria to host cells. They are also called microbial surface components recognizing adhesive matrix molecules.67,68 Gram positive organisms possess afimbrial proteins on their surfaces that presumably aid in binding to host cells. The three most commonly studied afibrillar adhesins are those that bind salivary glycoprotein, bind fibronectin, or are composed of lipoteichoic acid (LTA).68,69 Salivary binding proteins are commonly found in pathogens and commensals of the oral cavity. Both Streptococcus spp. and Actinomyces spp. possess these proteins. Fibronectin binding protein (FBP) is necessary for S. aureus invasion and binds both fibronectin and collagen to form a bridge between the FBP and the host cell integrin (integrin α5β1).70,71 The FBPs are essential for invasion of both epithelial and endothelial cells in S. pyogenes.67–68 Heterologs of FBP have been demonstrated in S. pneumoniae of humans, S. equi subsp. equi, and S. equi subsp. zooepidemicus. Other potential equine pathogens that have FDPs on their surface include Actinomyces spp. E. faecalis, and L. monocytogenes.69,72 Lipoteichoic acid (LTA), a common binding factor found in Streptococcus group A bacteria, is important in adhesion of bacteria to cells.73 This protein is also important in stimulation of cytokine secretion from the cells during infection and has been demonstrated in group B Streptococcus, including S. equi subsp. equi.74 A less commonly described afibrillar adhesin is composed of surface polypeptide chains in Corynebacterium that binds to lectin.75 Binding of these proteins can be abolished by trypsin treatment. Afibrillar adhesins are also present in gram negative organisms; the most commonly studied are conserved high molecular weight adhesion proteins of Haemophilus influenzae and Bordetella pertussis.73
Biofilm Ahesins
Bacterial ecology has emphasized the importance of biofilm for colonization of both biotic and abiotic surfaces of bacteria.59 A requirement for biofilm formation is a tight interaction of bacterial adhesins with surface receptors that promote further bacterial aggregation. The most important family of biofilm adherence proteins (BAPs) is that of Staphylococcus aureus. These proteins have high molecular mass and repetitive structures whose size and number can be varied during the course of infection, possibly allowing for immune evasion.
Cytoskeletal Changes
Binding of bacteria frequently results in cytoskeleton changes within the host cell in order to enhance susceptibility to invasion. “Membrane ruffling” is a virulence factor that results in either internalization or breakdown of intracellular components to allow for invasion of tissues. Once bacteria attach, the change in the membrane itself (ruffling) aids in colonization of host surfaces or even uptake of the bacteria. The most common virulence proteins of both gram positive and gram negative are bacteria lectins.63-76 Although attachment is thought to be the primary role of these proteins, attachment itself results in an intracellular change, including actin rearrangements, cell signaling regulation, or actual secretion of bacterial substances into the host cell. These proteins are highly conserved in bacteria and are important targets for immunoprophylaxis.
Membrane transformation for uptake of intracellular bacteria such as Yersinia spp., Listeria monocytogenes, Salmonella spp., and Shigella flexneri can be either zipperlike or triggerlike. Binding results in actin rearrangement and engulfment of these intracellular bacteria by both phagocytic and nonphagocytic cells. Yersinia and Listeria spp. form a zipperlike relationship wherein the tightly adhered bacteria result in actin polymerization and formation of a phagocytic cup through actin nucleation, polymerization, and depolamerization.77 Alternatively, Salmonella and Shigella bacteria adhere and secrete proteins that are translocated into the host cell cytoplasm and trigger actin polymerization.78 In addition to membrane ruffling, Mycobacterium avium and Salmonella spp. also rely on activation of intracellular GTPases, leading to phagocytosis.79,80
FACTORS THAT ENHANCE SPREAD OF BACTERIA
Survival in Host Environment
CAPSULE
It was observed as early as 1928 that engulfment and digestion of S. pneumoniae were associated with lack of capsule,81 and by 1940 it was clear that this virulence factor was genetically encoded.82 Early studies with S. equi subsp. equi demonstrated that resistance to phagocytosis was associated with an increase in capsule and M-protein,83 and in a model of S. equi subsp. zooepidemicus infection in mice, enhancement of virulence was associated with increased amount of capsule, which increased resistance to phagocytosis.84 Although colonization of the guttural pouch occurs with nonencapsulated S. equi subsp. equi strains, induction of lymphadenopathy is associated with capsular strains.85 In more recent studies of S. equi subsp. equi infection, rapid colonization in the lingual and pharyngeal tonsil is dependent on genetically associated virulence factors that control colony morphology, with the mucoid strain having enhanced virulence.33
Escherichia coli capsule is the “prototype” capsule of gram negative bacteria.86 Ultrastucturally, this capsule forms a fine fibrillar meshwork covering the bacteria surface.87 The K antigen is one of the major antigens, is temperature dependent in its antigenic structure and amount, 88 and is highly associated with increased pathogenicity and resistance to phagocytosis.89,90 The other major antigen for gram negative bacteria is the lipopolysaccharide (LPS) minus the lipid A component of the molecule. A third minor component, colanic acid, appears to exhibit antiphagocytic activity in some E. coli. Classically, the presence of capsule on E. coli prevents complement mediated phagocytosis.91 The LPS itself activates complement, and the capsule prevents immune activation by concealing the LPS molecule.92
Capsules of anaerobic bacteria are unique, and these structures may directly account for the formation of abscesses within the host. The capsule of B. fragilis has two distinct polysaccharides composed of repeating subunits with oppositely charged groups (Zwitter ion).93 This polysaccharide complex injected alone promotes the induction of abscess. Infection of rodents with the encapsulated form of Bacteroides and Fusobacterium spp. results in the formation of intraperitoneal abscesses, whereas nonencapsulated bacteria do not cause abscessation.94–96 Synergism of capsular anaerobes with other bacteria occurs; nonencapsulated bacteria have enhanced survival in abscesses and produce capsule when cultured or inoculated with encapsulated bacteria.95
ANTICOMPLEMENT FACTORS
Similar to and overlapping with capsule are structural proteins that block complement. The O side chain of LPS on gram negative bacteria is an anticomplement factor.97 The longer the side chain, the greater the distance between phagocytes and bacteria. The capsular component, sialic acid, interacts with O antigen to prevent the formation of C3 converatase.98 Bacterial enzymes are formed by Streptococcus spp. and other organisms that damage the polymorph chemoattractant, C5a.99,100 Production of a protein in Salmonella spp., encoded by the rck gene, prevents insertion of the C9 fragment of complement into the bacterial membrane.9 The M protein of S. equi subps. equi appears to decrease deposition of complement on the surface of bacteria.
RESISTANCE TO PHAGOCYTOSIS
Recent studies of streptococci have shown that when M-protein content is kept constant, the amount of capsule is actually correlated with resistance to phagocytosis.85 Resistance to in vitro phagocytosis can be abolished with treatment with hyaluronidase and induction of specific immunity against M-protein of S. equi subsp. equi and S. equi subsp. zooepidemicus.101 The M proteins of Streptococcus spp. are also essential for resistance to phagocytosis blocking complement.83,102–104 This mechanism for complement resistance appears to be through enhancement of binding of fibrinogen to the bacteria in the presence of M protein.105–107
PHAGOLYSOSOMAL SURVIVAL
The intracellular environment should be inhospitable for bacteria, yet many organisms are ingested by phagocytic cells and utilize this environment to multiply and disseminate. In normal phagolysomal fusion, the phagocytic vesicle first becomes fused with a host cell endosome. Shortly thereafter, fusion with the lysosome occurs. Several digestive proteins are released within the lysosome, and there is a decrease in pH, resulting in inactivation and digestion of a foreign protein or microorganism. Bacteria have devised ways to escape the phagosome or use the phagosome as a niche for extended survival. Shigella spp. and Listeria monocytogenes are bacteria that escape the phagocytic vesicle to multiply in the host cell cytoplasm.108 Before escape from the phagosome, Listeria modulates maturation of the phagosome by delaying fusion with the lysosome.109 Both Mycobacterium and Legionella spp. cause a change in the maturation of the phagosome leading to uninhibited replication within macrophages.110,111 Engulfment of Salmonella spp. in the phagosome appears to induce formation of an actual phagolysosome, but the organism survives acidification and reactive oxygen intermediates (ROIs), likely through production of catalase and superoxide dismutase.112,113 The acid tolerance response (ATR) is important primarily for intracellular bacteria that are able to withstand highly acidic environments, including L. monocytogenes, R. equi, and Salmonella spp.114,115 Genes that control virulence in Salmonella are actually upregulated by an acidic environment. Rhodococcus equi suppresses acidification of the phagolysosome in addition to inhibiting phagolysosomal fusion.116 Recently, homologous genes that regulate survival under high temperature and oxidative stress were also determined to be essential for phagolysomal survival in R. equi.117 Although the mechanism by which R. equi inhibits phagolysosomal fusion is unknown, opsonization by an R. equi–specific antibody results in enhanced fusion and killing.115,118 Resistance to phagolysosomal fusion and replication in the phagolysosome appears dependent on the presence of the 90 kDA virulence plasmid.114 Rickettsia, Neorickettsia (formerly Ehrlichia), and Rhodococcus bacteria appear to inhibit phagolysosomal fusion.
HOST SUBSTRATE UTILIZATION
Nutrition of bacteria is intimately associated with cellular and tissue environments. The proper level of iron is important because iron is required for the production of reactive oxygen intermediates. The fur gene in E. coli was first identified as the major regulator of iron acquisition.119 Homologs of this gene have been identified in many other bacteria, including Salmonella spp., Vibrio spp., Pseudomonas aeruginosa, S. aureus, and B. fragilis.119,120 Rhodococcus equi has its own chromosomally encoded genes (iupABC) that allow survival in low iron environments.121 In addition, bacteria possess siderophores, which are very potent chelators of iron.122 These siderophores are secreted outside of the bacterial cell, uptake iron, and are taken back up by the cells through a receptor-mediated process. After internalization the iron is cleaved and utilized by the bacteria. Resistance to carbon, nitrogen starvation, and new substrate utilization are also important means by which bacteria survive inhospitable environments. The latter has been demonstrated as important for R. equi survival in the phagosome, where bacteria become more efficient at membrane fatty acid and cholesterol utilization under anaerobic conditions.123
Damage to Host Tissues
TOXINS
Exotoxins are virulence factors secreted by the host that frequently function to aid spread of infection (Figure 2-3). Both gram positive and gram negative organisms secrete an array of exotoxins. Classically, there are three types of exotoxins.61 The first is the A-B form, in which there are two distinct parts to the toxin: one for binding and one for enzymatic action. The second does not appear to have distinct parts and forms pores within host membranes. The third is superantigen, which forms a bond between the major histocompatibility complex (MHC) class II receptor of macrophages, resulting in release of various T cell helper–mediated cytokine cascades. Several examples of common equine pathogens are discussed.
STAPHYLOCOCCUS AUREUS
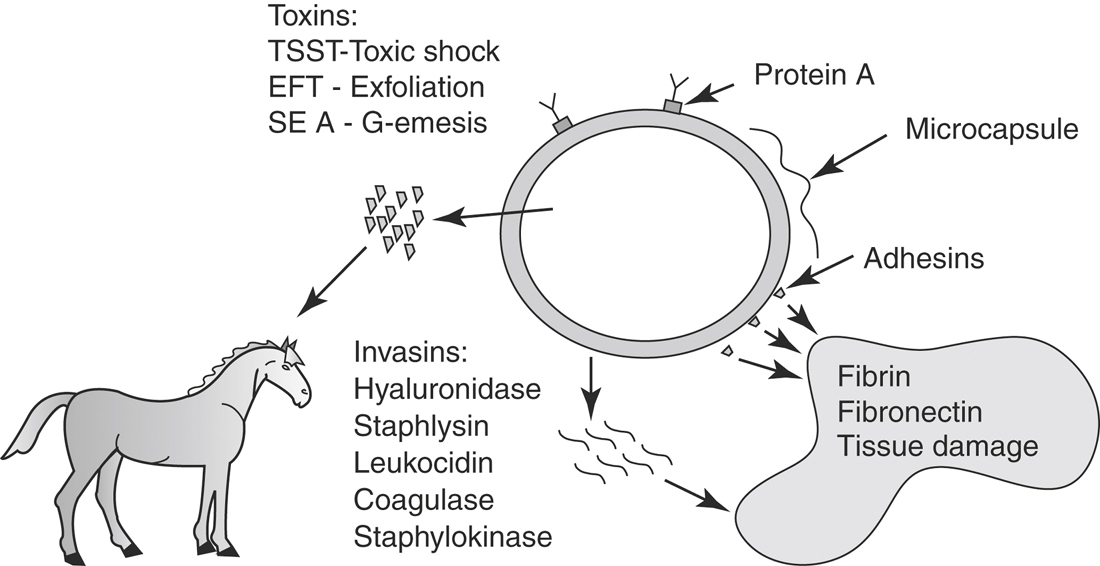
Toxins of Staphylococcus aureus are well characterized, provide examples of several types of toxins produced by the same organism, and are directly associated with the pathogenesis of this disease (see Figure 2-3).124 Although there are few overt syndromes recognized in the horse that are specifically caused by S. aureus, toxins that may be important for disease in the horse include the four hemolysins, toxic shock syndrome toxin-1 (TSST-1), exfoliative toxins, and leukocidin (Table 2-3). For example, cellulitis induced by S. aureus in the horse may be similar to wound-associated toxic shock in humans. Toxic shock syndrome is a disease of increased capillary permeability characterized by hypotension, hypoalbuminemia, and edema. The toxins of S. aureus work in concert to (1) induce massive release of cytokines, (2) increase sensitivity to cytokines, and (3) damage endothelial cells directly. The superantigen toxin of S. aureus forms a bridge between the MHC receptor on macrophages and the T cell receptor. This bridge results in a massive release of interleukin-2 with the downstream effect of T cell proliferation and induction of Type 1 cytokines that stimulate release of pro-inflammatory cytokines from macrophages. Exfoliative toxin, which induces T cell proliferation, and TSST-1 have been identified in equine S. aureus isolates associated with severe phlegmon and metritis.125,126
TABLE 2-3 Mechanisms of Virulence of Staphylococcus aureus
| Factor | Mechanism of Action |
|---|---|
| Toxic shock syndrome toxin | Hemolysin |
| Exfoliative toxin | Hemolysin, T cell proliferation |
| Leukocidin | Hemolysin |
| Superantigen | Cross linking and activation of macrophages and T cells |
| Staphylokinase | Plasminogen activator (lysis of fibrin clots) |
| α toxin | |
| β hemolysis (Sphingomyelinase C) | Toxic to erythrocytes |
| δ toxin | Erythrocytes, cellular |
| γ-hemolysin | Degranulation of leukocytes |
| Leukocidin | Degranulation of leukocytes |
| Panton-Valentine leukocidin | Pore former in white blood cells; skin necrosis |
| Arginine catabolic mobile elements | Increased colonization of community-acquired methicillin resistant Staphylococcus aureus (CA-MRSA) |
| PSM-alpha | Enhanced destruction of white blood cells in CA-MRSA |
The enhanced virulence displayed by community-acquired methicillin resistant Staphylococcus aureus (CA-MRSA) has been associated with the cytotoxin Panton-Valentine leukocidin (PVL), which is a β-pore former that causes enhanced spread through skin necrosis.127,128 PVL has been identified in almost all CA-MRSA strains, but a causal role in enhanced virulence is still subject to debate; recent work with this toxin was not proved in in vivo models.129 Other toxins, such as arginine catabolic mobile elements (ACMEs) and phenol soluble modules (PSMs), are under intense scrutiny. Deletion of ACME from MRSA strains greatly decreased in vivo virulence, and acquisition of this genetic element likely confers a more robust ability to colonize over non-ACME strains, giving rise to an epidemic-prone bacterial clone.128–130 The PSM-αprotein demonstrates enhanced destruction of white blood cells.127
CLOSTRIDIUM INFECTIONS
Clostridrium bacteria mediate clinical disease by producing exotoxins. The Clostridium bacteria causing botulism and tetanus excrete neurotoxins to which horses are highly susceptible. The main toxins of botulism (BNT) and tetanus (TNT) are remarkably similar even though both toxins exert effects that appear to be polar opposites, with botulism causing a flaccid paralysis and tetanus a spastic paralysis.131 Both toxins share amino acid sequences of metalloproteinases that are most similar to zinc-requiring endopeptidases. With botulism there are six serologically different neurotoxins, with most reports in horses caused by type B toxin, although A, C, and D have also been identified.131–135 There is only one tetanus neurotoxin that is responsible for the clinical signs of spastic paralysis.
Neurotoxin is secreted as a progenitor and must be cleaved by proteases (trypsin) to the derivative toxin to produce clinical signs. The active toxin consists of a light and heavy chain (A-B type toxin) that mediates paralysis by blockage of acetylcholine esterase in a three-step process.61,136 The first step is the rapid step, which is involved in recognition by the neurotoxin of the receptor on the nerve and rapid binding. In the second, translocation occurs as the active site of the molecule is internalized into the nerve cell ending by endocytosis. The acidified vesicle induces a conformational change in the toxin so that it can be translocated to the cytosol. The third and final step is the slow step, wherein cell neuroproteins called synaptopeptidases are cleaved and acetylcholine release prevented. Neurotoxin cleaves the proteins synaptobrevin (SNAP), synaptosome associated membrane proteins (VAMP), and syntaxin, which are involved with neurotransmitter release, exocytosis of the neurotransmitter vesicle, and vesicle-cell membrane fusion, respectively. The inactivation of one or more of these proteins accounts for the prevention of release of synaptic vesicles. The different botulinum neurotoxins exhibit variable specificity to these proteins. Types B, D, F, and G are active against VAMP, whereas A, C, and E are active against SNAP, and C is the only form that cleaves syntaxin.
The difference in clinical signs noted between the C. tetanus and C. botulinum is a reflection of the binding and level of activity of the respective toxin. Clostridium botulinum binds to peripheral nerves and C. tetanus to cells within the CNS. Further, C. botulinum toxin binding results in prevention of downstream release of other neurotransmitters, such as γ-aminobutyric acid. Injection of C. tetanus toxin intravenously in low doses results in flaccid paralysis.
The main pathologic features of disease cause by C. perfringens are edema, necrosis, and death regardless of the site of action.136 Toxins elaborated by these clostridia include both lethal and nonlethal toxins that cause necrosis and hemolysis and frequently contain lecithinases and lipases. Clostridium perfringens has four major lethal toxins, including an enterotoxin and three minor toxins. Thus far genetic characterization of equine isolates associated with diarrhea indicate that the majority are type A, the toxic effects of which are mediated in part by α-toxin.137 The cpe-toxin or enterotoxin has been detected by enzyme-linked immunosorbent assay (ELISA) in approximately 16% to 19% of equine isolates, so disease has been linked to both enterotoxigenic type A and nonenterotoxigenic type A strains.21,138 There are individual case reports of neonatal diarrhea associated with Type B (β and ε toxins), Type C (β toxin), and Type D (ε toxin) strains that also carry the α toxin.139,140 Table 2-4 is a summary of the mechanisms of actions of the toxins of Clostridium perfringens.136,141–145
TABLE 2-4 Mechanisms of Virulence of Clostridium perfringens Toxins
| Factor | Mechanism of Action |
|---|---|
| α | Zinc metalloproteinase: hemolysis, lethality through cardiovascular collapse and death, disruption of endothelium and erythrocytic cell membranes by hydrolyzation of lecithin and sphingomyelin, platelet aggregation. Stimulates the release of massive amounts of inflammatory cytokines. Activation of coagulation, aggregation of red blood cells, which occurs throughout body, including muscle cells, which results in myositic syndromes |
| β | Massive catecholamine release with drop in heart rate and blood pressure. Forms pores especially in neurons, resulting in neurotoxicity |
| ε | Pore former within neuronal cell membrane, resulting in irreversible cell membrane damage and death |
| Enterotoxin | Change in ion movements of enterocytes affecting cellular metabolism |
| β-2 | Mechanism of action in dispute; associated with enteritis of large animals |
Clostridium difficile has five toxins, although disease causation is thought to rely on the production of toxins A and B.146,147 The evidence to date indicates that both toxin A and B mediate the pathogenesis of C. difficile diarrhea.147 Research suggests that the formation of the classic pathologic lesion, the pseudomembrane, is a result of the combined actions of toxin A, toxin B, and interleukin-8. Classically, toxin A is considered an enterotoxin and lethal, whereas toxin B is a virulent cytotoxin. Toxin A appears to be the primary mediator of fluid accumulation; injection of toxin A results in fluid accumulation, cell necrosis, and recruitment of inflammatory cells.148,149 One mechanism of this fluid accumulation appears to be by interruption of actin filaments and destruction of tight junctions. Toxin A also results in neutrophil recruitment, and evidence suggests that this is mediated through the direct action of the toxin on macrophages to release interleukin-1, tumor necrosis factor α, and leukotrienes. In cell culture toxin A can cause interleukin-8 secretion (a potent recruiter of neutrophils), cell detachment, and apoptosis of separated cells. This direct activation of macrophages and secretion of IL-8 is through a calcium- and calmodulin-dependent mechanism that results in direct nuclear upregulation after nuclear translocation of transcription factors NF-κβ and AP-1.148 Toxin B is more cytotoxic than toxin A, especially to human epithelial cells.
CELL DEATH
Apoptosis is a distinctive morphologic process that results in cleavage of nuclear material and scavenging of unwarranted cells without immune activation.150 Apoptosis or programmed cell death is an important pathway for complex organisms to deal with damaged and diseased tissue. Apoptosis avoids the release of the tissue-damaging enzymes and nonspecific elimination of tissue that occurs in cellular necrosis. Several bacteria have modulated the host apoptotic pathways for enhancement of survival.151 Shigella flexneri, S. typhimurium, and toxins of S. aureus, Pseudomonas spp., and C. diphtheriae have demonstrated programmed cell death as a consequence of cellular infection or exposure.150,152 The protein of S. flexneri, IpaB, induces apoptosis by binding to and activating the cellular enzyme caspase 1, which induces apoptosis of macrophages.108 Staphylococcus aureus α toxin, which is similar to listeriolysin O (LLO) presumably escapes the macrophage after engulfment and induces host cell apoptosis.124 The TSST toxin of S. aureus induces B cell apoptosis and blocks immunoglobulin production.
PATHOGENESIS OF FUNGAL INFECTIONS
Of the 250,000 species of fungi, fewer than 200 are true pathogens.1 Superficial mycoses affect the hair shaft and the superficial epidermis. Cutaneous mycoses (dermatophytosis) infect the epidermis, dermis, hair, and nails of animals, and Microsporum, Trichophyton, and Epidermophyton spp. are the most commonly associated pathogenic genera. Subcutaneous tissues can become infected with Sporothrix, Conidiobolus, Basidiobolus spp., and members of the Dematiaceae fungi, including the Chromoblastomycosis, Mycetoma, and Phaeohyphomycosis spp. infections. Most of these infections are introduced by penetration through skin or opportunistic invasion of damaged skin surfaces. Histoplasma capsulatum, Coccidioides immitis, Blastomyces dermatitidis, and Paracoccidioides brasiliensis are the four most important fungal pathogens that can cause systemic infection. The most common opportunistic infections include Candida albicans, Aspergillus spp., Cryptococcus neoformans, Mucor spp., and Pneumocystis carinii.
FACTORS THAT ENHANCE ENTRY OF FUNGI
Adherence
Fungal virulence factors may be more complex than those of bacteria because of the higher degree of opportunism that occurs with a change in host status. There may be subtle factors that, combined with host status, result in a certain fungus attaining a virulent state. For instance, the typical fungal wall is composed of three major polysaccharides: mannose; β-1,3 and β-1,6 linked glucans; and chitin. Chitin mutants in C. albicans are less virulent when tested in rodent models than are wild-type fungi.153 Further, a mutant C. albicans that cannot synthesize complex mannose oligosaccharides does not adhere to other yeast and epithelial cells and has lost virulence in a guinea pig model.154 Both of these mutants can proliferate in vitro normally, and whether or not this is an actual virulence factor is unclear.
As with bacteria, cellular adherence is an important prerequisite for infection and colonization of the host. Adhesins have been identified in C. albicans and B. dermatitidis. Two genes have been associated with adhesion in C. albicans. The first is a glycoprotein that has sequences consistent with agglutinating activity. Transformation of this gene into other nonadherent fungal species results in adhesion of the transformed yeast to cells.155 Candida albicans also has integrin-like proteins, the disruption of which results in diminished hyphal growth, adhesion to cells, and loss of virulence in mice156,157 The B. dermatitidis adhesin mediates binding to human monocyte-macrophages through the CD14 receptor.158
FACTORS THAT ENHANCE SPREAD OF FUNGI
Evasion of Immune Defenses
CAPSULE
Many fungi have polysaccharide capsules that, like bacteria, help resist phagocytosis and immune activation. The capsule of C. neoformans inhibits leukocyte accumulation, cytokine secretion, and macrophage phagocytosis.159 Mutants without capsule are highly infective and avirulent. As indicated earlier, many fungi are engulfed by macrophages, and intracellular survival is mediated by virulence factors. Macrophages are decimating cells for C. albicans,160 H. capsulatum,161 and B. dematitidis Histoplasma capsulatum is primarily a yeast in vivo, and this form infects macrophages. Phagolysosomal fusion occurs at a normal rate,162 but blockage of acidification of the phagolysosome occurs.163
Adaptation to Host Environment
MORPHOLOGY AND TEMPERATURE
Adaptability to the host environment is also a trait that enhances fungal virulence. Fungal dimorphism, which is the ability to adopt another morphologic state, is clearly tied to virulence. Mutants of C. albicans that cannot switch to the hyphal form are avirulent in certain in vivo models, although both forms likely contribute to pathogenesis.164 A second adaptation is called phenotypic switching, in which colonies of fungi change during in vitro growth. Candida albicans, C. neoformans, and H. capsulatum all display phenotypic switching, and these different phenotypes are associated with degrees of virulence.165,166 Histoplasma capsulatum spontaneously gives rise to mutants that have less capsule, are less virulent, and are not cytotoxic to macrophages.167 The signal for dimorphic fungi to change form is usually temperature change. Many fungal species germinate within the host with increased temperature, allowing for dissemination within the host. Calcineurin is found in many yeast and mammalian cells, and in C. neoformes this protein mediates the ability of this fungus to grow at 37° C.168 Temperature and heat shock are also mediated by the calcium-dependent protein cyclophilin B in Aspergillus.169 Adaptation to mammalian pH is genetically controlled in C. albicans. Mutants display abnormal cell morphology at physiologic pH ranges.170
NUTRIENTS
Nutrient requirements that affect virulence include primarily melanin, iron, and calcium. Melanins are present in the wall of C. neoformans, and melanin can scavenge ROI intermediates, making the organism resistant to the oxidative burst of neutrophils.171 Pathogenic fungi have siderophores and high affinity ferric iron reductase to acquire iron from low iron environments.172,173 Histoplasma capsulatum secretes a calcium-binding protein that enhances calcium uptake from calcium-poor environments.174,175 Without this protein H. capsulatum cannot form colonies and does not survive in cultured macrophages.
DAMAGE TO HOST TISSUES
Toxins
Exposure to both pathogenic and saprophytic fungi is an everyday occurrence. Respiratory contamination and infection are important for many pulmonary species, but skin penetration and dissemination from necrotic gut are important portals for large animals also. Dissemination after the initial infection is dependent on previous damage to host tissues, deeper mechanical penetration, or actual invasion of new tissues. C. albicans can actually grow through and replace cell membranes.160 True molds invade blood vessels and grow along the intima of the vessels. Fungi secrete many degradative enzymes, including proteinases, phosphatases, and DNAses in order to surmount structural barriers.176 A group of genes called secreted aspartyl proteinase (SAP) genes allow more persistent colonization of host surfaces and deeper penetration.177
When C. immitis invades the host, the fungi form endospores. These endospores secrete a proteinase and a urease that likely aid in the breakdown of pulmonary tissues.178–180 The two proteinases of A. fumigatus break down elastin, a major component of lung tissues.181,182 Phospholipase activity has been demonstrated in C. albicans, C. neoformans, and A. fumigatus.183 Strains of Candida spp. with high amounts of this enzyme have higher virulence,184 and abolishing this activity results in decreased adherence of the organism.185 Host eicosanoids enhance fungal colonization. Recent evidence demonstrates production of eicosanoids by both dermatophytosis and systemic fungi.186
Apoptosis
Fungi induce apoptosis, which may be due either to the direct effect of a fungal toxin or secondary to host cell cytoskeleton rearrangements.187 The gliotoxin of A. fumigatus can induce DNA fragmentation and apoptosis in macrophages.188 This toxin also has many other immunosuppressive qualities, which include inhibition of the neutrophil respiratory burst and T cell activation.
MECHANISMS OF ESTABLISHMENT AND SPREAD OF VIRAL INFECTIONS
Despite the great significance of some viral infections, recognizing that many equine viruses are ubiquitous, weakly pathogenic, or not associated with any known disease under normal circumstances is also important. Some examples include equine adenovirus,1 respiratory and enteric reoviruses2 (the term reo is derived from the acronym for respiratory enteric orphan, indicating that these isolates have not been associated with disease), and equine herpesvirus (EHV) type 2.3 Some host-virus relationships may be mutualistic in that virally derived genetic elements are theorized to benefit the host by facilitating genetic variability and evolution.4 Thus many viruses are of no practical clinical significance, and no control efforts are warranted. For this reason, the veterinarian should never assume that the recovery of a virus from a clinical specimen is significant without proof that the virus can cause the disease in question.
Veterinary virology is a rapidly changing field. New diseases continue to emerge or be discovered; a dramatic example is the Hendra paramyxovirus that appeared in Australia in 1994, killing horses and human beings.5 More recently, another paramyxovirus, the Salem virus, has been identified in the United States, although the clinical importance of this virus is unclear.6 Less dramatic, but of more relevance to most equine veterinarians, is the association of bovine papillomavirus with sarcoids.7,8 A number of equine diseases occur in which viral involvement has yet to be excluded, including Theiler’s hepatitis9 and lymphosarcoma.10 Viral origins also likely will be discovered for diseases in which viruses were not suspected previously to play a role; in human beings and mice, viruses have been proposed to have a role in everything from diabetes to obesity.11,12 However, the greatest advances in veterinary virology are in understanding the molecular biology of viral replication, virus-cell interactions, and virus-host interactions. Related advances in molecular biology techniques, such as PCR and immunohistochemistry, also are providing sensitive, specific, and rapid tools for the diagnosis of viral infections. In the realm of antiviral drugs, aggressive searches are ongoing for effective and economical drugs for treatment of human beings, and in the near future antiviral drugs likely will be a realistic therapeutic option for equine veterinarians.13,14 Finally, significant advances in the design of vaccines likely will improve the efficacy of immunization.
This chapter outlines the general ways that viruses cause disease and describes the most important virus-cell and virus-host interactions that result in pathologic conditions. Although the focus is on equine viruses whenever possible, the principles described are generally not species dependent, and no attempt is made to limit the discussion to recognized equine viral pathogens or diseases. Discussion of a particular mechanism or virus also should not be taken to suggest that this mechanism or type of virus has been documented in horses. When possible, supporting references have been selected to include review articles or texts for additional information about key concepts.
VIRUSES AND VIRUS-CELL INTERACTIONS
An in-depth discussion of viral structure, taxonomy, and replication is beyond the scope of this chapter, and the reader is referred to textbooks of veterinary or human virology for more detailed information.15,16 However, a brief overview is presented to emphasize those features that have clinical relevance.
Virus Structure, Taxonomy, and Replication
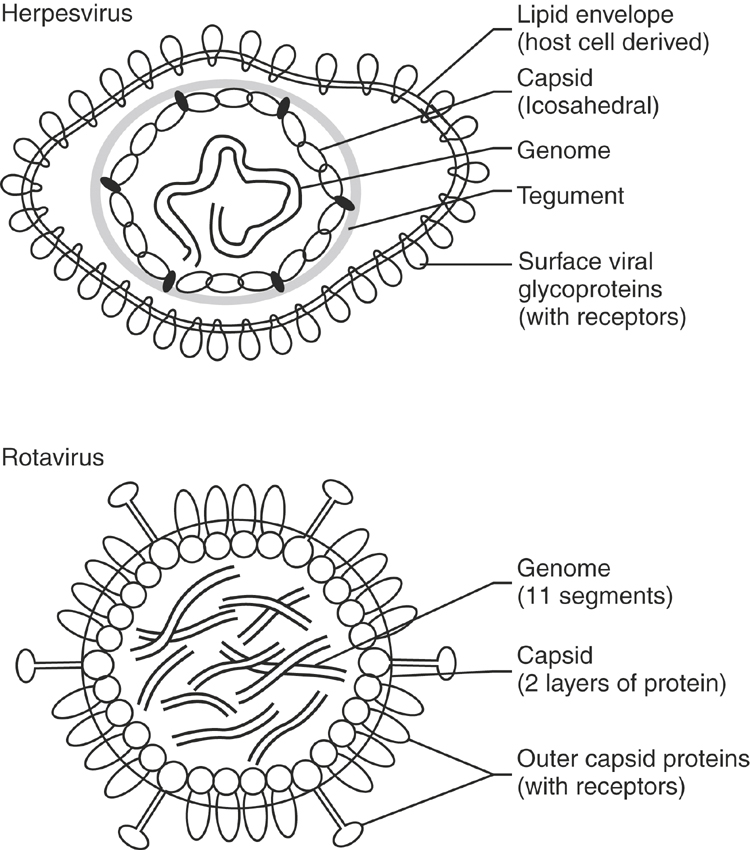
The fundamental structure of all viruses is a DNA or RNA genome enclosed by a coat of protein called the capsid (Figure 2-4). For viruses that are enveloped, the capsid is enclosed further by a host cell–derived lipid membrane into which viral proteins have been incorporated. In addition to protecting the viral genome, the capsid and other associated structural proteins (e.g., matrix proteins) are important for virus assembly, packaging the viral genome, releasing the genome into a target cell, and for nonenveloped viruses, providing receptors that bind to host cells. For enveloped viruses the receptors are incorporated into the lipid membrane. The primary clinical significance of these features is that enveloped viruses, because of their fragile lipid membrane, are highly susceptible to inactivation by heat, desiccation, or detergents, and transmission typically requires direct exchange of body fluids, short distance aerosols, or arthropod vectors. In contrast, nonenveloped viruses (e.g., equine rotavirus) are resistant to physical inactivation, and environmental contamination is more likely to be a significant factor in their transmission.