CHAPTER 11 MARSUPIALS
HUSBANDRY
Fat-Tailed Dunnart
Fat-tailed dunnarts (Sminthopsis crassicaudita) can be maintained in windowless rooms with artificial light and at a constant temperature of 20° C to 24° C. Multiple rooms can be designated, each with a particular purpose. One room can have a reduced day length of 8 hours in preparation for stimulating males to breed. Other rooms to include are a nursery/maintain room and a breeding room—each with a 16-hour-day length. Galvanized metal breeding cages (∼50 ×35 ×20 cm) with hinged mesh tops, removable plate glass fronts, and metal base trays are advisable for this species. Nest boxes should be plastic (for ease of cleaning) and well ventilated, with shredded paper as a substrate. Animals that are able to eat on their own can be housed in standard laboratory rat cages. Plastic mouse wheels should be provided for breeding animals but not for nursing animals. It is essential to provide dry, clean autoclaved sand in cage trays for animals to groom themselves.
PERFORMING A PHYSICAL EXAMINATION
The diversity of marsupial species presented to the veterinary practitioner appears to be increasing. Before initiating a clinical examination on any marsupial species, it is the responsibility of the practitioner to be familiar with the normal appearance and behavior of the species he or she is examining.
In the wild, sugar gliders are communal as a species. Each individual is marked with the scent of the dominant male in the group, and his scent is also used to mark territory (Figure 11-1). Sugar gliders will feed on sap, in particular, gum from the wattle tree, as well as fruit, nectar, and insects procured during flight. They can be aggressive animals and will attack small mammals if they perceive those mammals as a threat.
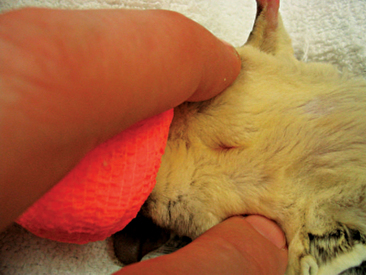
Male sugar gliders have a scent gland on top of their heads; however, owners may report it as a bald spot and think it is the result of the animal’s rubbing its head on the cage, nest box, or directly onto the female. These animals also have a scent gland on their chest (see Figure 11-1). Male sugar gliders, like other marsupials, have unique genitalia. The penis of these animals is bifurcated, and the scrotum is located cranial to the penis (Figure 11-2).
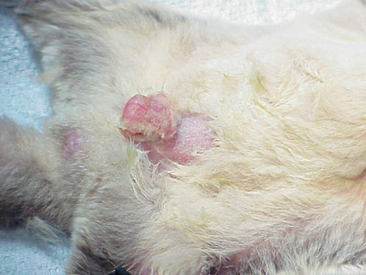
Figure 11-2 Pendulous scrotum of male sugar glider. The penis is actually located candal to the scrotum.
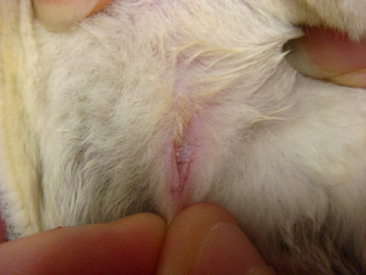
Abdominal masses may indicate that a female glider is pregnant. The symmetry of these masses can reveal the number of young being carried by the female (Figure 11-3). Most pregnant sugar gliders give birth to one or two joeys. Gestation length is a mere 16 days, and once born, the joeys find their way to the pouch at the earliest stages of development. Joeys will remain in the pouch up to 2½ months. Thereafter, the developing young will begin to venture from the pouch, returning primarily to nurse and for protection. The intermittent pouch life will last for a period of 1 to 2 months. The male glider is quite gentle and receptive to these newcomers of the nest box.
Rear leg paresis or paralysis has been noted within the species. The exact cause(s) remain undetermined, although reduced opportunity for exercise as a result of captivity may play a role. Nutritional deficiencies, such as inadequate protein intake, should be considered, and injections of vitamin B complex, vitamin C, vitamins A and D3, vitamin E and selenium, and calcium may be administered to treat the condition.1
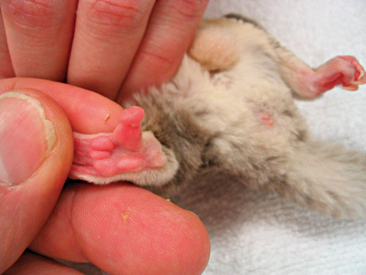
Instability in, or absence of, normal stance should be investigated, commencing with a physical exam. General anesthesia of the patient should be used to ensure a thorough examination of the animal is obtained. Primary muscle bellies should be checked for asymmetry as a result of atrophy or swelling. Digits should be palpated for injury, infection, or foreign material (Figure 11-4). The appendages should be examined for joint symmetry and mobility, flexion and extension. The spinal cord should be palpated for deviation. Radiographs will serve to assist in determining soft tissue or skeletal injury. Appropriate nutrition and husbandry can often help to reduce the incidence of many of these locomotor abnormalities, and as such, client education is essential.
ANESTHESIA AND RESTRAINT
Manual Restraint of Macropods
Adult macropods of smaller size, 5 to 10 kg (11-22 lb), may be captured using a net on a long pole (1-2 m). The net should be composed of a fine mesh or solid cloth to prevent entanglement or leg injuries. Macropods sensing capture will often run along the fence line of an enclosure. A keeper can take advantage of this flight response by taking a position along the fence line. A space should be maintained between the fence line and the person that is poised to capture the animal. The capture pole with attached net is held parallel to the fence but behind the keeper. Other individuals then herd the selected animal along the fence line toward the net. As the animal passes between the keeper and the fence, the net is brought forward in front of the animal and the animal contained. Personnel involved with capture and restraint should be cautious not to overstress the animal, as stress may result in hyperthermia and subsequent exertional myopathy. If the macropod is not captured on the first attempt, consider postponement of the procedure or darting the animal. Once the animal is netted, basic clinical procedures can be performed. In animals that weigh 36 kg (80 lb) or greater, sedation by remote injection techniques is the safest approach for capture, reducing injury risk to both humans and animal.
Chemical Restraint of Macropods
Medetomidine-ketamine and tiletamine-zolazepam are two chemical immobilization combinations reported to be particularly effective. Both therapeutic combinations provide a rapid and smooth induction within 10 minutes. The advantage of medetomidine-ketamine over tiletamine-zolazepam is its reversibility with atipamezole. Dosages utilized for these combinations are species dependant. Doses of 40 mg/kg of medetomidine mixed with 4 mg/kg of ketamine administered intramuscularly (IM) have been found to be an effective combination when used on eastern gray kangaroos.1 Ranges of 40 to 70 mg/kg of medetomidine with 4 to 7 mg/kg of ketamine have been found to be effective for red kangaroos, wallaroos, Dorcopsis wallabies (Dorcospsis macleayi), and Goodfellow’s tree kangaroos (Dendrolagus goodfellowi).1 Atipamezole given at a dose 5 times the total mg of administered medetomidine has been found to be effective for reversal in the above-mentioned species.1 A dose of 100 mg/kg of medetomidine and 5 mg/kg ketamine IM has been effective in immobilizing red necked wallabies.1
Tiletamine-zolazepam at a dose of 2 to 8 mg/kg is recommended for chemical immobilization of tree kangaroos.1 In most macropods, however, the general dose range is 3 to 11 mg/kg IM.1
Sugar Glider Anesthesia
Anesthesia for sugar glider patients is best accomplished with inhalation agents (e.g., isoflurane). A medium- or large-sized mask can be used as an induction chamber, with isoflurane being delivered at a level of 5% and an oxygen flow rate of 1 L/min (Figure 11-5). Once induced, the animal can be transferred to a smaller face mask and maintained at 2% isoflurane (600-800 ml/L oxygen). Placement of an endotracheal tube (e.g., 14-16 gauge intravenous catheter) may be attempted, but it presents an obvious challenge in terms of the animal’s size and the possibility of tube blockage caused by the buildup of respiratory secretions.
Injectable anesthetics are rarely used to anesthetize sugar glider patients because inhalation agents have a much reduced risk and are easily provided. One study reported using tiletamine-zolazepam at doses ranging from 8.4 to 12.8 mg/kg IM with no adverse side effects.1 However, tiletamine-zolazepam at 10 mg/kg has been associated with neurologic problems and death in squirrel gliders (Petaurus norfolcensis), so caution is advised with use of this drug.1 Ketamine has also been used to induce anesthesia in gliders at a recommended dose of 20 mg/kg.1 Acepromazine has been combined with butorphanol and given orally or with ketamine and given subcutaneously (SC) to maintain postoperative sedation and analgesia.
Manual Restraint of Opossums
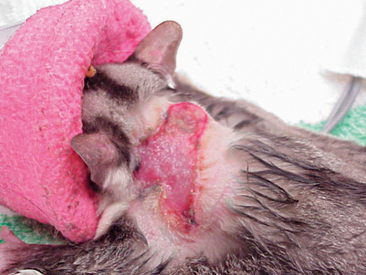
The private practitioner or wildlife rehabilitator may be presented with a Virginia opossum (Didelphus virginiana) (Figure 11-6). Often, these animals have incurred vehicle or fight injuries, and as a result, attention and care must be taken in their restraint (Figure 11-7). Heavy gloves and towels should be used during the removal of the animal from its enclosure. These animals have particularly sharp teeth and, if not appropriately restrained, will turn quickly to bite. Similarly, opossums retain long claws and may scratch in a threatened situation. A firm grasp of the scruff of the neck and the base of the tail are essential in the movement of the animal. If assistance is available, these restraints can be divided between two individuals. However, if the handler is alone, the animal’s head and neck should be held farthest from the handler and the base of the tail held as the primary means of support in the carriage of the animal. As in other species, the tip of the tail should never be held, as injury may result. Masked induction with 5% isoflurane and 1.5 L/min of oxygen followed by endotracheal intubation and a maintenance rate of 2% to 3% isoflurane and an oxygen flow rate of 1.5 L/min of oxygen is considered sufficient for basic examination, wound care, and diagnostics.

Figure 11-6 Opossums may be brought to veterinary hospitals as nonreleasable pets or injured wildlife.
COMMON PRESENTATIONS BY SYSTEM
Respiratory System
FOREIGN BODIES
Foreign bodies in the nares manifest in symptoms of unilateral purulent nasal discharge, sneezing, and persistent pawing at the nares. The offending foreign body must be visualized before removal. The animal first should be heavily sedated or placed under general anesthesia. An otoscope, rigid arthroscope, or endoscope can then be used to locate the foreign material. Removal is best achieved with alligator forceps. Antibiotics should be prescribed as a prophylaxis against bacterial infection. Clavulanic acid and amoxicillin (Clavamox) can be given to macropods and wombats at a dose of 12.5 mg/kg IM twice a day.1 Alternatively, a Clavamox dose of 25 mg/kg can be given IM or SC once daily. The oral administration of Clavamox in macropods and wombats has been associated with yeast overgrowth in the gastrointestinal tract and subsequent diarrhea; therefore, if Clavamox is prescribed, treatment and patient response should be carefully monitored.
VIRAL INFECTIONS
A poxvirus is associated with the development of papillomatous proliferations on the skin of macropods. The areas affected most often by these papillomatous proliferations are the head and extremities. The development of papillomas around the external nares and mouth may interfere with normal respiration. Marsupial species affected by this condition include tammar wallabies, Agile wallabies (Macropus agilis), swamp wallabies (Wallabia bicolor), eastern wallaroos (Macropus robustus), quokkas, red kangaroos (Macropus rufus), eastern gray kangaroos (Macropus giganteus), and western gray kangaroos (Macropus fuliginosus).1 The disease itself is considered self-limiting, but if the growths impair an animal’s ability to eat or breathe, surgical removal is an option.
An orbivirus was reported as causing death in Australian tammar wallabies during the summer months.2 No premonitory signs were evident, and animals were usually found either comatose or dead. Diagnosis was based on history, serologic testing, and postmortem examination. Gross pathologic findings included marked pulmonary congestion and edema, hepatic congestion, and frequently, subcutaneous edema in the hindlimbs.2 No effective treatment is currently available. Vaccine development for this orbivirus is currently in progress.
A herpesvirus has been established as the cause of death in a variety of marsupial species.2 Clinical signs associated with this virus include respiratory rales, conjunctivitis, incoordination, and death.2 Serologic testing is available, but a definitive diagnosis is often made when histopathologic examination of affected liver tissue reveals intranuclear inclusion bodies in hepatocytes. A viable treatment option has yet to be found.
BACTERIAL INFECTIONS
Although bacterial infection of the upper respiratory tract is uncommon in marsupial species, the koala appears to be most susceptible. Bacterial infections reported to cause rhinitis in the koala include Bordetella bronchiseptica, Pseudomonas spp., Corynebacterium spp., Streptococcus spp., Escherichia coli, Micrococcus spp., Staphylococcus spp., Proteus spp., Pasteurella spp., and Enterobacter spp.1 In the koala, Chlamydophila psittaci has been implicated as a cause of nasal discharge.1 Clinical signs of upper respiratory tract disease are not unlike those seen in mammalian species. Bilateral nasal discharge, sneezing, anorexia, and coughing are the more common signs encountered. Nasal discharge can be clear or mucopurulent in appearance. Pharyngitis and regional lymph node enlargement have been seen to occur. If Chlamydophila psittaci is suspected, specific guidelines should be followed for specimen collection, transport, and storage. The practitioner should be aware of the following:1
Swabs of the nasal mucosa can be analyzed with antigen enzyme-linked immunosorbent assay (ELISA) to assist in the diagnosis of chlamydiosis. Antigen ELISA kits are commercially available to practitioners. Antigen detection through direct immunofluorescence can also be performed with a commercial kit, but this technique appears less sensitive than the ELISA.1
Effective treatments for chlamydiosis include doxycycline hydrochloride, 5 mg/kg every 7 days IM for 6 weeks; chloramphenicol, 30 mg/kg SC every 12 hours for 7 to 10 days; and oxytetracycline, 5 mg/kg SC every 7 days for a maximum of 4 injections.1 Pseudoephedrine hydrochloride at a dosage of 1 mg/kg every 12 hours can be administered via nebulization or per os (PO) as a decongestant. The mucolytic, bromhexine hydrochloride given at a dosage of 2 mg/kg every 12 hours PO or through nebulization also can help to relieve clinical signs.1 Supplemental feeding should be given to koalas when they are being treated with antibiotics, as antimicrobial effects of the therapeutic agents on the gastrointestinal flora have been associated with significant weight loss.
Necrobacillosis, commonly known as lumpy jaw, is a tooth root infection frequently encountered in captive macropods. Several factors are believed to foster the development of the disease, including overcrowding and poor husbandry—which lead to excessive fecal contamination, especially around feed stations—and a diet containing soft feed or awns. Bacteroides (Dichelobacter) nodosus and Fusobacterium necrophorum, usually acting synergistically, invade and cause infection. Other bacteria less commonly isolated from tooth root infections include Actinomyces spp. and Corynebacterium spp. Additional opportunistic bacteria will invade as an infection advances and may be the only organisms isolated when cultures are finally obtained. Primary clinical signs associated with necrobacillosis are swelling around the face and jaw, excessive salivation, loss of condition because of difficulty in eating, depression, and lethargy. Proptosis of the globe and squinting may be observed as a result of retrobulbar abscessation. A bacteremia may result, spreading organisms to other areas of the body, including the spleen, liver, stomach, bones, and lungs. Treatment of lumpy jaw is focused on removal of affected teeth and debridement of affected soft tissue. Clindamycin is the antibiotic of choice at a dose of 11 mg/kg PO (capsules or liquid) every 12 hours. Animals find the liquid formulation more palatable than capsules, and an injectable formulation exists but is expensive and restricted to IV use. Duration of treatment should be a minimum of 6 months because of the possibility of disease recurrence. Large doses of penicillin (150 mg/kg procaine penicillin and 112.5 mg/kg benzathine penicillin) every second day for approximately 2 weeks has also been recommended, but efficacy of this treatment course is unknown.1
Prevention of necrobacillosis focuses on providing appropriate diet and husbandry for captive animals. Overcrowding of macropod species should be avoided. Environments should be routinely cleaned to avoid the buildup of fecal material both on the ground and around food sources. A Bacteroides nodosus vaccine has been recommended at the following weights and doses: less than 5 kg, 0.2 ml SC; 5 to 10 kg, 0.25 ml SC; 10 to 20 kg, 0.5 ml SC; and more than 20 kg, 1 ml SC.1 The skin over the ribs is the preferred site for recommended SC vaccine injection. Sterile abscesses have been reported at the vaccine injection sites. The vaccine schedule is 2 doses 4 weeks apart, then once a year.
Mycobacteriosis has been reported as a source of respiratory disease in marsupial species.1 Tuberculosis caused by Mycobacterium bovis has had particular impact on the brushtail possum population in New Zealand. The disease is contracted from possums grazing in pastures frequented by infected animals (e.g., cattle, deer, or other possums). Discharge is emitted from the lymph nodes of these diseased animals and usually serves as the source of pasture contamination. Once a possum is exposed, the organism spreads throughout the body, targeting the lungs and other vital organs. Clinical signs associated with marsupial tuberculosis include dyspnea, depression, anorexia, and weight loss. Treatment of the wild brushtail possum is often not pursued because this animal is considered a pest species in New Zealand. The brushtail possum is viewed as perpetuating Mycobacterium bovis in cattle and deer herds in New Zealand, resulting in significant economic loss.
Mycobacteriosis has had a similar impact on captive tree kangaroos in the United States. It has been reported as a common cause of disease and mortality in three species of tree kangaroos in particular, including Matschie’s (Dendrolagus matschiei), Goodfellow’s (Dendrolagus goodfellowi), and Grizzled (Dendrolagus inustus).1 Mycobacterium avium complex is the cause of more than 90% of infections diagnosed in captive tree kangaroos maintained in the United States. The apparent susceptibility of these animals is attributed to a cellular immune response comparatively lower than what is observed in eutherian mammals or other marsupial species. Clinical signs associated with mycobacteriosis in tree kangaroos include weight loss, dyspnea, lameness, abscesses, neurologic problems, and blindness. Definitive antemortem diagnosis is a challenge with the tree kangaroo, and signalment, history, clinical signs, radiology, and transtracheal washes are included as part of the recommended diagnostic regimen. The treatment protocol for infected tree kangaroos is amikacin, 3 mg/kg IM every 12 hours; rifabutin, 20 mg/kg PO every 24 hours; ethambutol, 20 mg/kg PO every 24 hours, and azithromycin, 20 mg/kg PO every 24 hours.1 The duration of treatment can be quite long, with amikacin usually administered for 10 to 12 weeks and oral medications for 3 years or longer. Unfortunately, treatments have yielded poor results. The mycobacterial organism can often still be detected on cultures of transtracheal washes performed 3 or more years after treatment was initiated. Preventing animals from exposure to the organism is the best approach to controlling this organism. Captive tree kangaroos should not be housed near captive birds because of the risk of transmission posed by Mycobacterium avium complex.
FUNGAL INFECTIONS
One of the more common infections affecting the upper respiratory tract of marsupial species is cryptococcosis. Australian marsupials including brushtail possums, ringtail possums (Pseudocheirus peregrinus), striped possums (Dactylopsila trivirgata), Leadbeater’s possums (Gymnobelideus leadbeateri), tammar wallabies, red-necked wallabies (Macropus rufogriseus), spectacled hare wallabies (Largorchestes conspicillatus), quokkas (Setonix brachyurus), and wombats and echidnas (Tachyglossus aculeatus) have been reported as having the disease.1 The species that appears particularly susceptible, however, is the koala. The causative agent is the yeast-like fungi, Cryptococcus neoformans var gattii. The organism’s close association with river red gums (Eucalyptus camaldulensis), mugga ironbarks (Eucalyptyus sideroxylon), and other red gum species results in extremely high koala exposure rates. Inhalation of the organism from a contaminated environment is believed to be the primary source of exposure. Clinical signs that have been observed in the koala include unilateral or bilateral nasal discharge, sneezing, and anorexia. The usual course of infection starts at the nares and progresses into the sinuses, with secondary lung involvement. Granulomatous formation may occur in the respiratory tract. Once the animal’s respiratory system is infected and there is upper respiratory disease, the organism can infect the central nervous system (CNS) as well. With CNS infection, neurologic signs (e.g., seizures, blindness, behavior changes) may be observed. One of the primary diagnostic tests used to diagnose this disease is the microscopic examination of stained smears collected from the nasal cavity. Diff-Quik staining of the collected sample is easily performed and offers excellent visualization of the yeast-like organisms. India ink may be used as an alternative. Nasal discharge should be cultured, as the organism will grow on Saboraud’s agar at 25° C and 37° C. A latex-cryptococcal antigen test (LCAT) is available to detect soluble cryptococcal capsular polysaccharide antigen in body fluid samples. The LCAT can be a particularly effective test when cryptococcosis is suspected, but the site(s) of infection is (are) unknown. Serial dilutions of serum are combined with a suspension of latex particles coated with hyperimmune rabbit serum, with titers of 1 : 2 or greater considered positive. The strength of a positive response can be an indicator of disease severity and, ultimately, prognosis for treatment response and recovery. The decline in LCAT titer is usually slower when compared with clinical improvement but can still be an effective tool in indicating treatment success and prognosis. Survey radiographs of the nasal cavities, sinuses, and lungs also will assist in localizing areas of infection, and survey computed tomography (CT) scans offer even more precision if available.
The recommended treatment protocol for cryptococcosis is fluconazole, 3 mg/kg PO every 24 hours for 30 to 60 days, with a loading dose of 6 mg/kg.1 Amphotericin B is administered in approximately 50 to 60 ml of Hartmann’s solution or 0.9% NaCl at a dose of 0.5 mg/kg SC every second day for 30 days because of the potential for renal toxicity that exists with amphotericin B.1 Renal function should be checked every 2 weeks. If fluconazole is cost prohibitive, ketoconazole is an acceptable alternative. The dosage offered for ketoconazole is 10 mg/kg PO every 12 hours for 30 to 60 days.1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree