CHAPTER 34 Leptospirosis
Leptospirosis is a bacterial disease of worldwide distribution caused by spirochetes of the genus Leptospira. The disease affects humans, domestic animals, and wildlife, including reptiles and amphibians. The first formal report of leptospirosis was in human patients by Adolf Weil over 100 years ago, accounting for the name Weil’s disease.1,2 Subsequently, leptospirosis was identified in dogs and livestock. The first report of naturally occurring leptospirosis in horses was in 1947 in Russia.3 In horses the disease has most often been associated with abortion and equine recurrent uveitis; however, sporadic cases of renal and hepatic disease have also been reported.
ETIOLOGY
The order Spirochaetales includes two families of spiral bacteria, Spirochaetaceae and Leptospiraceae, which share unique morphologic and functional features4,5 (Fig. 34-1). The genus Leptospira is within the family Leptospiraceae and includes a large number of both pathogenic and nonpathogenic bacteria. Morphologically, all the leptospires are indistinguishable and are flexible, tightly coiled, unicellular bacteria. At least one end is hook shaped, leading Stimson6 in 1907 to name the organism initially Spirochaeta interrogans because of the resemblance to a question mark. The organisms are slender, with a diameter of approximately 0.1 to 3.0 μm and a length of 6 to 20 μm. The helical coil amplitude is 0.1 to 0.15 μm.
Structurally, the leptospires have a helical protoplasmic cylinder consisting of nuclear material, cytoplasm, and the cytoplasmic membrane and peptidoglycan cell wall4,7 (Fig. 34-2). There are two axial flagella, or axial filaments, each attaching at opposing ends of the organism by platelike insertion discs. The distal end of each flagellum is not attached and extends toward the center of the cell, sometimes overlapping the flagellum from the opposite end. These flagella facilitate motility. As with other spirochetes, the leptospires have a double-membrane structure in which the cytoplasmic membrane and peptidoglycan cell wall are closely associated and are overlaid by an outer membrane that surrounds the protoplasmic cylinder and periplasmic flagella. This outer membrane is rich in lipopolysaccharide, the composition of which is similar to that of other gram-negative bacteria, but with less potent endotoxic activity.8,9 Despite the gram-negative characteristics of leptospires, they do not stain well with conventional bacteriologic dyes. Therefore, other techniques, such as darkfield microscopy, silver impregnation, and immunologic staining, have been developed for identification of leptospires.
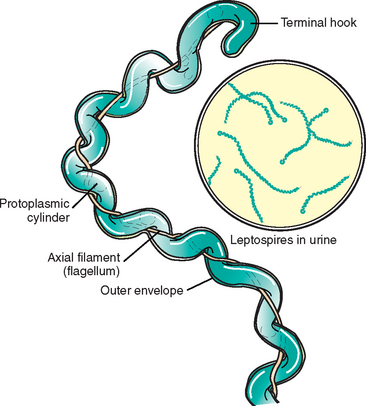
Fig. 34-2 Ultrastructure of pathogenic leptospires.
(From Greene CE, editor: Infectious diseases of the dog and cat, ed 3, St Louis, 2006, Saunders, p 404; courtesy University of Georgia, Athens.)
Leptospires are obligate aerobes with an optimum growth temperature of 28° to 30° C (82.4°-86° F).4 Although slow growing, they can be isolated on artificial media, a characteristic that is unique among spirochetes. They require either long-chain fatty acids or long-chain alcohols as a primary energy source.
The leptospiral genome is approximately 5000 kilobases (kb) in size and consists of two sections, a 4330-kb chromosome and a smaller 359-kb chromosome.10–12 No other plasmids have been identified. Evidence suggests horizontal transfer of genetic material within the genus. The genome has been sequenced, and a number of leptospiral genes have been cloned.
In the traditional serologic classification system, the leptospires are grouped into two species, the pathogenic species Leptospira interrogans and the nonpathogenic, saprophytic species Leptospira biflexa.4,5,12 Within each species, the “serovars” are organized into “serogroups” based on shared antigenicity. In this system, L. interrogans contains at least 218 serovars organized into 23 serogroups, and L. biflexa contains at least 60 serovars organized into 28 serogroups.
Analysis of leptospiral DNA by DNA hybridization revealed significant genetic heterogeneity within the two phenotypic species, L. interrogans and L. biflexa, resulting in the reclassification of species based on DNA homology.13–15 Currently, 17 “genomospecies” of Leptospira have been defined, and ongoing studies suggest that further taxonomic revisions are likely.14,16–19 Serologic testing is used within the genomospecies to identify serovars (Table 34-1). The name L. interrogans is used to identify species in both the phenotypic and the genotypic classification systems, causing some confusion. Therefore, L. interrogans sensu stricto, meaning “in the strict sense,” is sometimes used to denote the specific genomospecies, whereas L. interrogans sensu lato, meaning “in the broad sense,” is used when referring to the more general phenotypic species.
Table 34-1 Genomospecies Identified with Selected Serovars of Leptospira
| SEROVAR | GENOMOSPECIES |
|---|---|
| Australis | interrogans |
| Autumnalis | interrogans |
| Bratislava | interrogans |
| Canicola | interrogans |
| Grippotyphosa | interrogans, kirschneri |
| Hardjo | interrogans, borgpetersenii, meyeri |
| Icterohaemorrhagiae | interrogans, inadai |
| Pomona | interrogans, noguchii |
| Sejroe | borgpetersenii |
EPIDEMIOLOGY
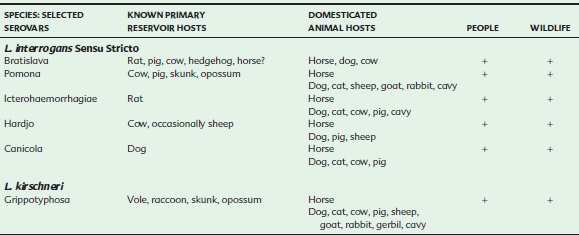
Leptospirosis is maintained in nature by subclinically infected maintenance hosts, also known as reservoir hosts or definitive hosts.4,5,12 These maintenance hosts, which include numerous wild and domestic animal species, serve as a source of infection for incidental or accidental hosts, including humans (Table 34-2). Specific serovars of Leptospira are generally found in particular maintenance host species, and epidemiologic studies suggest that these host preferences may vary both with geographic regions and over time. In the maintenance hosts the organism is considered to be host adapted, and infection is endemic. Although the host is highly susceptible to infection, the organism is usually of low pathogenicity, and disease is usually subclinical or mild. There is generally prolonged excretion of leptospires in the urine, making these maintenance hosts the primary source of environmental contamination and transmission to other species.
In contrast, incidental hosts typically have a low susceptibility to infection, but they are more likely to develop acute, severe disease when they do become infected. Incidental hosts generally shed organisms for relatively short periods, making them inefficient transmitters of disease. For most serovars of Leptospira, horses are incidental hosts. However, recent evidence suggests that as with cattle and pigs, horses may be a maintenance host for L. interrogans serovar Bratislava.20–25
The primary source of exposure to Leptospira is infected urine. Maintenance hosts may shed organisms in the urine for prolonged periods because of chronic infection of the renal tubules.12,26–28 The organism can also be spread through aborted fetuses, placental tissues, uterine discharges, and milk. Contact with the organism may occur either directly or indirectly through contaminated soil, bedding, feed, or drinking water. Once in the environment, leptospires may survive for several weeks under favorable conditions, which is generally a warm, moist environment, such as stagnant or slow-moving warm water. Survival is favored by a neutral or slightly alkaline pH and inhibited by a pH of less than 6 or greater than 8. Therefore, organisms can survive only transiently in undiluted acidic urine. Ambient temperatures of 10° to 25° C (50°-77° F) favor survival, and temperatures lower than 7° to 10° C (44.6°-50° F) or higher than 34° to 36° C (93.2°-96.8° F) are detrimental. The organism is susceptible to freezing and drying. Some evidence indicates that leptospires may survive in insects or other invertebrate hosts, but the significance of this is unknown.29,30
Although contact with infected fluids is the most common means of direct transmission of leptospirosis, venereal and transplacental transfer have been documented in some species.12,26,28 Leptospiral organisms have been found in the semen of infected bulls, and transmission by natural breeding or artificial insemination can occur but is uncommon. In humans, transmission through sexual intercourse during convalescence has been reported.31 Data in horses are limited, but there are currently no reports of leptospiral transmission through semen or embryo transfer.32 Fetal infection has been documented in foals after localization in the pregnant uterus, resulting in resorption, abortion, stillbirth, or weak neonatal foals.20,21,33–38
Seroprevalence
Serologic surveys to assess the prevalence of antibody to leptospiral organisms have been performed in multiple species, including horses. These studies confirm that there is widespread exposure to Leptospira worldwide and that exposure is significantly more common than clinical disease. Specifically, in horses, seroprevalence has varied from 1% to 95% depending on the geographic location and the serovars assessed.20,22,23,39–60 In some studies, horses were more likely to be seropositive than other domestic animal species.42,47,49,50 Titers to a wide variety of serovars have been reported in horses, and although there is variation between studies, in general, titers to Leptospira interrogans serovars Icterohaemorrhagiae, Bratislava, Pomona, Ballum, and Grippotyphosa tend to be most common. Various serovars of leptospires have also been isolated from bacteriologic surveys of horses at necropsy.34,51,59 Studies in The Netherlands, Northern Ireland, Canada, and Kentucky suggest that at least in these regions, horses may be a maintenance host for serovar Bratislava because of the prevalence of this serovar.20–25
Risk Factors
Several studies have assessed risk factors for exposure to leptospirosis in horses. An early study in Australia did not find any correlation between increased moisture in the environment and seroprevalence.60 In a Canadian study of 1923 horses in which the seroprevalence ranged from 0.8% to 94.6% depending on the serovar, age was significantly associated with the presence of titers, with the chance of being seropositive increasing by approximately 10% with each year of life.48 In addition, horses managed individually, such as horses at a track, were about half as likely to be seropositive as those managed in groups, such as rodeo horses. Several other studies have also shown an increase in prevalence with increasing age.25,41,61,62 Barwick et al.61,62 evaluated sera from 2551 horses on 572 farms in the state of New York and used a multidimensional indexing system to identify risk factors associated with seropositivity to various serovars. Although findings varied somewhat depending on the specific serovar assessed, age was again identified as a risk factor, as were management practices. The density of horses turned out together was positively associated with the risk of exposure. Also, exposure to rodents, wildlife, and potentially contaminated soil and water could be associated with increased risk of seropositivity to some serovars.
Abortion, Stillbirths, and Neonatal Deaths
Well established as a cause of abortion in cattle and swine, leptospirosis has been sporadically reported as a cause of abortion in horses since the 1950s, but problems in making an accurate diagnosis have made determination of the true prevalence difficult.36,63,64 Several recent studies indicate leptospirosis is a significant cause of abortion in mares.35,65–70 In a study reviewing the pathology case records of 3527 cases of abortion, stillbirth, and perinatal death in horses, fetoplacental infection caused by bacteria was diagnosed in 628 cases, of which leptospirosis was identified in 78.69 In comparison, equine herpesvirus was diagnosed in 143 cases. Related studies in Kentucky have found leptospirosis in 2.5% to 4.4% of submissions of equine fetuses, stillborn foals, and placentas.65–67 Abortions, stillbirths, and perinatal death have also been confirmed in other geographic locations worldwide.34,35,37,71
PATHOGENESIS
The usual portal of entry for leptospires is by penetration of the mucous membranes and abraded or soft, moist skin. Occasionally, organisms may enter by inhalation, ingestion, or animal bites. As with most infectious diseases, the outcome of exposure to leptospires depends on the dose, virulence, and host susceptibility. After entry into the lymphatics and bloodstream, the organisms multiply and are carried to multiple organs. Bacteremia typically occurs 4 to 10 days after initial exposure. There is no apparent tissue tropism, and leptospires replicate in many tissues, including the kidneys, liver, spleen, central nervous system (CNS), eye, mammary gland, and genital tract. The action of the axial flagella and the release of hyaluronidases may facilitate invasion, especially into the CNS and eye. Fetal infection can occur following localization in the pregnant uterus. The organism may persist, most often in renal tubular epithelial cells.
The exact mechanisms by which leptospires cause disease are not completely understood. One feature of the disease is systemic vasculitis. Studies in mice suggest that the endothelial cell membrane of small vessels is disrupted by the intercalation of a glycolipoprotein toxin that displaces host long-chain fatty acids required to maintain vascular cell wall integrity.72 The damage to the vascular endothelium allows for further migration of spirochetes into the tissues, as well as capillary leakage and hemorrhage, with disruption of tissue architecture, ischemia, and necrosis. There is a broad spectrum of clinical signs depending on the specific tissues affected and the severity of the infection.
Clear differences exist in the virulence of different isolates of Leptospira, but information relative to specific virulence factors and their role in pathogenesis is limited. Several outer membrane proteins, including lipopolysaccharide (LPS), may contribute to virulence. Highly immunogenic, LPS is responsible for the serovar specificity of leptospires.12,73 Although leptospiral LPS exhibits endotoxic activity in biologic assays, it is of low potency.8,9,74–76 Outer membrane components may play a role in the pathogenesis of interstitial nephritis.8,77,78 In addition, the outer envelope may have an antiphagocytic function.79
A number of factors in addition to the outer membrane proteins potentially contribute to the virulence of leptospires. Toxins other than LPS are produced by some pathogenic strains. Several serovars, including Pomona and Hardjo, produce hemolysins.80,81 Protein and glycolipoprotein cytotoxins have also been identified.12 Virulent leptospires can induce apoptosis in vitro and in vivo, and increased concentrations of inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), are found in patients with leptospirosis.82
Immune-mediated mechanisms may also play a role in the pathogenesis of leptospirosis in several species. This is supported in part by the observation that significant clinical disease may be present even after apparent clearance of the majority of organisms.12 In human patients, levels of circulating immune complexes correlate with disease severity, and in patients who survived, circulating immune complex concentrations decreased concurrently with clinical improvement.83 A number of autoantibodies, including antiplatelet antibody, have been detected in clinical leptospirosis, but their role in pathogenicity is uncertain.12 In horses, evidence indicates that immune mechanisms may be important in some cases of equine recurrent uveitis, as discussed later.84–88
The humoral immune response appears to be primarily responsible for the control of infection and immunity to leptospires.12,89,90 Antibodies, which are predominantly directed against outer envelope epitopes, are generally produced within a few days of infection. Immunity usually is specific to the inciting serovar and closely related serovars, although some broadly reactive antigens have also been described. After opsonization by antibodies, organisms are cleared by the reticuloendothelial system. However, when there is infection by a host-adapted serovar in a maintenance host, concentrations of antibody may remain low, allowing organisms to persist, primarily in the kidney. Fetal infection, especially in the third trimester, may result in a specific antibody response that can be protective. Passive immunity can be transferred by antibodies alone, but cell-mediated immune responses to leptospires also occur.12 However, these cell-mediated responses are currently thought to have a minimal role in protection. The duration of immunity to leptospirosis is uncertain.
The pathogenesis of leptospiral-induced inflammation in equine recurrent uveitis (ERU) has not been fully elucidated despite considerable research in this area. There is evidence that both persistent infection and autoimmune mechanisms may play a role in the pathogenesis. Leptospira within the eye may cause direct damage, initiate a local immune response to the bacteria, or incite an autoimmune reaction through molecular mimicry. Studies investigating the cellular and cytokine response to Leptospira, as well as immunopathology, support a role for immune mechanisms in the pathogenesis or ERU, in particular a delayed-type hypersensitivity response.84,91
Horses inoculated with L. interrogans produce cross-reactive antibodies that bind to cornea and lens in addition to Leptospira.85–88,92 After inoculation with killed leptospiral organisms, antibodies reacting with cornea have been found in tears, aqueous humor, and serum of horses.86 In addition, horses inoculated with either killed Leptospira or equine cornea develop cross-reacting antibody and corneal opacity.85 Specifically, a 90-kilodalton (kDa) leptospiral protein has been identified that shares antigenicity with a 66-kDa equine corneal protein.93 This leptospiral protein responsible for antigen mimicry appears to be present in several serovars of L. interrogans sensu stricto, but it is not present in the nonpathogenic L. biflexa sensu stricto or in L. borgpetersenii serovar Tarassovis strain Perepelicin, which is pathogenic but has not been associated with ERU.94 It has been hypothesized that persistent infection may not be necessary to induce immune-mediated ocular inflammation, because any release of inflammatory cytokines could reactivate memory T cells that react with ocular antigens.84,95
CLINICAL FINDINGS
Clinical leptospirosis in horses has been primarily associated with abortion and ERU, although sporadic cases of systemic disease have also been reported. After experimental induction of leptospirosis in horses, fever was the most consistent clinical sign, with anorexia, listlessness, and icterus observed in some cases.96–98 The majority of experimentally infected horses exhibited leukocytosis and neutrophilia during acute infection, and hyperbilirubinemia was occasionally seen. Although not a consistent finding, some horses developed uveitis, generally several months after exposure.
Abortion, Stillbirth, and Neonatal Disease
Leptospiremia may lead to infection of the reproductive organs, and in pregnant animals, this may result in fetal resorption, abortion, stillbirth, or weak neonates. In an outbreak of leptospirosis associated with flooding on a farm with 70 broodmares, there were eight abortions, a stillbirth, three neonatal deaths, and a case of neonatal illness in a 6-week period.71 Abortion has also been associated with mixed Leptospira and equine herpesvirus type 1 (EHV-1) infection.67,99 Leptospiral abortions most often occur in middle to late gestation. Although affected placentas may appear grossly normal, they more frequently are thick, edematous, and hemorrhagic. The chorionic surface may appear brown and mucoid; occasionally the allantois is discolored green with adenomatous hyperplasia. Placental lesions are diffuse, consistent with placentitis secondary to leptospiremia.
Equine Recurrent Uveitis
Uveitis has long been recognized as a sequela of leptospirosis in both equine and human patients. In horses, uveitis has been described after naturally acquired infection as well as experimental infection with leptospires. Although the condition generally occurs months to years after infection, some cases may be more acute.100,96–98,101–105 Several serovars have been associated with uveitis, with Pomona being most often incriminated. Despite the association between ERU and leptospirosis, one should remember that most exposure to leptospires does not result in uveitis, and that uveitis may have many other causes.103,106,107
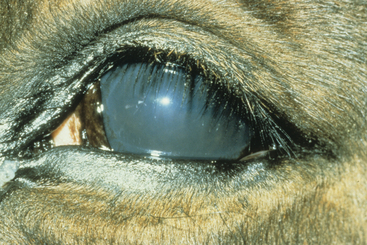
The signs of ERU include miosis, blepharospasm, photophobia, aqueous flare, iritis, ciliary injection, and occasionally keratitis (Fig. 34-3). Chronically, there may be synechia, cataract formation, atrophy of the corpora nigra, chorioretinitis, altered iris color, and ultimately blindness. These clinical signs are nonspecific and do not distinguish cases of leptospiral uveitis from those with other causes. Interestingly, a study by Dwyer et al.106 of 112 horses with ERU suggested that leptospiral uveitis may be more severe than ERU from other causes, because horses with uveitis that were seropositive to leptospires were 4.4 times more likely to lose vision than were seronegative horses with uveitis.
Systemic Disease in Adults and Foals
Although exposure to leptospires is common in the equine population, systemic disease appears to occur infrequently. However, clinical leptospirosis has been sporadically reported in horses of all ages. In several reports of leptospirosis in neonatal and premature foals, infection was most likely acquired in utero.33,35,64,71,108 Signs included weakness and icterus, and in one foal, hematuria and dysuria were present. Leptospirosis has also been diagnosed in slightly older foals. Leptospires were isolated from the urine of a 5-week-old foal with signs of dullness and poor body condition on a farm on which multiple foals had died by 14 weeks of age.109 Also, leptospirosis was thought to be involved in the deaths of 12 foals 4 to 12 weeks of age on one farm over a 3-year period.24 These foals had severe respiratory distress with depression and pyrexia. Other signs included jaundice in one foal, an unsteady gait in one foal, and diarrhea in two foals. The disease was rapidly fatal, and hemorrhagic pneumonia was found in all foals on necropsy. Leptospira interrogans serovar Lora was isolated from the blood of one foal, two foals had high microscopic agglutination test (MAT) titers in single sera, and two others had marked increases in the MAT titers in paired sera.
Reports of leptospirosis in adult horses have described fever, anorexia, and lethargy.107,110,133 Icterus and hepatic dysfunction have also been reported.45,101,104 Acute renal failure associated with leptospirosis has been documented in a 7-year-old stallion, a 3-month-old filly, and a 7-month-old filly.111–113 Common signs in these cases included anorexia and lethargy, and two of the three horses were febrile. Laboratory evaluation revealed leukocytosis with hyperfibrinogenemia, azotemia, and isothenuria. Ultrasound revealed enlarged kidneys in two of the three cases. A renal biopsy was only performed in the 3-month-old foal and confirmed tubulointerstitial nephritis.112 All affected horses responded to antimicrobial therapy.
DIAGNOSIS
Direct Detection Methods
Leptospires are difficult to visualize by standard light microscopy because they stain weakly or not at all with traditional stains. Therefore, darkfield microscopy is the most common means of microscopic identification.4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree