Fig. 1.1
Heart from left site (a) and right site (b). a artery, lig ligament, v vein, vv veins
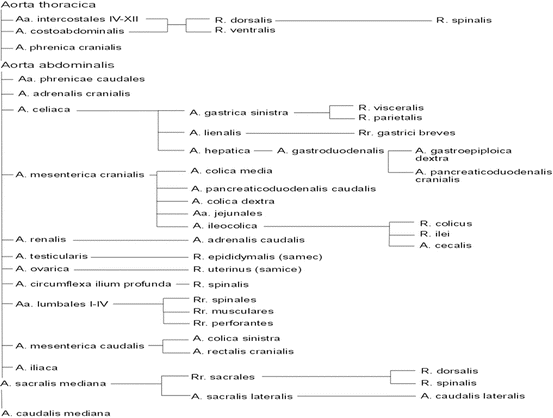
Fig. 1.2
Table of branches of thoracic and abdominal aorta. A artery, Aa arteries, R branch, Rr branches

Fig. 1.3
Scheme of gastrointestinal tract from dorsal site. a artery, aa arteries, r branch

Fig. 1.4
Arteries and veins from dorsal site of abdominal cavity. a arteries, lig ligament, ln lymph node, v vein
Sometimes incorrectly referred to as black rats or albino black rat in the Czech language, laboratory rats are usually an albino form of the brown rat (Rattus norvegicus varietas alba). The laboratory rat was domesticated and bred from initially wild-ranging brown rats (Rattus norvegicus norvegicus). Brown rats came into Europe from East Asia in the same way as did the black rat, i.e. with the assistance of humans. For wild-ranging brown rats, sewers, places under the ground, as well as commercial premises and human dwellings form the habitat. Unlike the black rat, brown rats become faster adapted to laboratory conditions. In this country, brown rats occur throughout the territory. Easily confused with black rats, with this species however ranging locally only in north-western Bohemia, particularly in the region of Polabská nížina (the Elbe Basin), brown rat’s most famous groups are three strains from which most of the other strains evolved by crossing: the Wistar rat (Philadelphia; W), Sprague Dawley (Madison, Wisconsin), and Long-Evans (LE), the last mentioned differing with its black-coloured head, neck, and the dorsal part of the body, the “hood” (often incorrectly called “capuchins”). Strains, however, may feature essential differences between each other [6].
Brown rats are omnivores, hunting even other smaller animals. Their body length being about 21–27 cm, the bare squamous tail measuring 17–23 cm, brown rats are utilised as experimental animals in labs in several forms, of which pure white with red eyes is the most common, followed by the black or yellowish-white version with black eyes. The hallmark of laboratory rats is their tameness and low biological variability. Unlike mice, brown rats are more resistant to infectious diseases. Under good welfare, their lifespan can be about 3 years. The weight varies according to diet, adult animals being able to reach 400 g or more. They reach sexual maturity in 3 months.
1.2 External Description and Determining the Age of Laboratory Rats in the Postnatal Period
Laboratory rat ’s body is elongated with a relatively small rostrum-shaped head. The body is covered with long stiff hairs except the nose, tail and load-bearing pads on the limbs. In albino strains, eyes possess an iris of pink colour, with eyelids clearly developed. The body is elongated in the movement, while bow-bent in the rest position. The tail is long (approximately 85 % of the body length), covered with horny scales under which there is a cover of sparse and indistinctive short hairs. Under the root of the tail there is anus. In males, there is scrotum at the tail root, which contains loosely placed testicles that may be present even intra-abdominally. Cranially before the scrotum, there is raised ostium preputiale, where one can palpate fine preputium and a penis in it. In females, there is a vaginal entry with a faint vulva anterior to the anus. Close below its ventral commissure, there is evident preputial sac with a distinct ostium preputiale, where one can pull a clitoris. On the basis of clitoris, there is an opening of urethra into the preputial sac. The terminal of the urinary tract in females is thus separated and even more clearly isolated from the genital organs than in laboratory mice. At the base of the trunk in lactating females, there are very well noticeable six pairs of milk nipples ventrally in the thoracic, abdominal and groin regions. The thoracic limbs are shorter and weaker than the pelvic limbs. They are used, among others, for digging, holding food and wiping the hair on the body. The thoracic and pelvic limbs have five fingers/toes. Both palmar and plantar section of the autopodium of the limbs are bare.
At birth, the newborn is bare. Palpebral fissures and external auditory canal are closed with an epithelial plug. Teeth are not yet erupted. Hair growth begins on day 2 to day 3. On day 4, the external auditory canal opens. On day 8 to day 10, incisors erupt. Eye slits open between day 10 and day 17. On day 12 to day 14, the young animal starts to eat solid food. On day 18, both upper and lower M1 erupt, followed by M2 on day 21. This is the time of weaning. M3 erupt on day 35. The initial coat is replaced in females on day 36 to day 38, whilst in males this occurs on day 36 to day 40. Around day 40, the process of descent of the testes is complete. Between day 35 and day 45, females begin their first ovulation. On day 42, the juvenile is fully independent. Sexual maturity for both sexes is reported to arrive within day 35 to day 90 of postnatal development. On day 40 to day 70, the epithelial plug of the vagina disappears in females. Laboratory rats reach the adult size on month 4 to month 6 [3, 6, 10].
1.3 Blood System and Anatomy of the Heart
Cor – located rather to the left of the median plane ventrally at the level of rib 3 to 6/7. The tip can be achieved in interchondral space 6. The size is about 2 cm per 1 cm. The lateral projection of the heart reaches cranially to the caudal margin of costa III, ventrally to the sternum and dorsal-caudally to the caudal periphery of costa V and its cartilage. In its dorsoventral projection, the heart extends cranially to the plane between the caudal margin of Th4 and the middle part of the second sternebra. Caudally, its extension is defined by the plane lying between the caudal margin of Th8 and the caudal end of the fourth sternebra. Apex cordis is directing slightly to the left. The brown rat heart weight varies from 0.9 g (for body weight of 200–300 g) as much as 1 g (body weight up to 450 g) in males, whilst in females the range is from 0.67 g (body weight 170–200 g) to 0.8 g (body weight of up to 280 g). Out of the total weight of the body, the heart makes around 0.289 % [3]. The heart structure is generally the same as in other placental mammals, with just a few minor differences to be noted. Right v. cranialis cava enters sinus venosus of the right atrium craniodorsally (v. cava cranialis is doubled in the brown rat), v. cava caudally, left v. cranialis cava from the left and two cardiac veins from the right, ventrally. Sinus venosus is separated from the atrium by two valves formed by the muscle of the sinus wall. Ventricles stretch almost equally to the tip of the heart. Papillary muscles of the left ventricle are represented by two strong strips of muscle that extend, from the cranial and caudal views, from the lateral wall of the ventricle. Right ventricle papillary muscles are usually made only as a conical ridge. In the walls of both ventricles there are numerous vv. cordis minimae, the occurrence being greater in the right ventricle. In the right ventricle, they are usually found in the interventricular septum, while in the left ventricle they are enclosed in the base of the papillary muscles. Cardiac ganglia occur in the region surrounding the terminal of the aorta and truncus pulmonalis, and then around the opening of venae cavae and in the wall of the atrium. The atrioventricular node is located in the interatrial septum near the right atrioventricular valve. The accessory atrioventricular node is normally present near the beginning of the aorta. The horseshoe-shaped sinoatrial node is located at the entrance of v. cava cranialis. A part of the fibrous skeleton of the heart is adjacent to the atrioventricular node; bypassing the right atrioventricular orifice, it runs between the opening and the aorta to form a complete fibrous ring around the left atrioventricular orifice. Subsequently, the atrial muscular system plunges into the fibrous base. The fibrous ring near the aorta contains cartilage, which may partly become calcified at month 6, this being furthered as the animal is getting older. Blood supply to the heart is one of a double system, meaning that in addition to the coronary arteries, parts of the heart are supplied by so-called accessory arteries. The fundamental arteries are a. interventricularis, a. coronaria dextra, a. coronaria sinistra and a. cardiomediastinalis. A. cardiomediastinalis is an accessory cardiac artery that comes, to the right, from a. thoracica interna (49 % of cases), from a. subclavia (31 % of cases), a. carotis communis (3 % of cases), or, as a separate cardiac branch, from a, thoracica interna (17 % of cases). On the left side, this artery comes from a. thoracica interna (89 % of cases), from a. subclavia (10 % of cases) or a. intercostalis suprema (1 % of cases) [1].
The aorta begins with the ascending aorta (aorta ascendens) which exits cranially to the heart . While still inside the pericardium, it produces coronary arteries that run in the ventrolateral direction. The aorta is then penetrating pericardium at the level of Th4, forming the aortic arch (arcus aortae) pointing left with a peak at approximately Th2. The aorta then runs caudally as the descending aorta (aorta descendens). Approximately 10 mm from the terminal of the aorta from the aortic arch, a. anonyma is exiting to branch, after about 4 mm of its course, into right a. subclavia and right a. carotis communis. Shortly after the peak of the aortic arch, left a. carotis communis is produced and a branch of left a. subclavia is formed immediately afterwards. The branching of each of the arteries in the laboratory rat is evident from the enclosed tables [3].
Due to frequent experimental interventions in the abdominal cavity, a more detailed description is provided of the aorta abdominalis branching. After aorta thoracica passing through the diaphragm in hiatus aorticus, the abdominal part of the aorta (aorta abdominalis) runs between the median arms of the diaphragm pillars, to some extent, near the cranial end of lumbar vertebra 2, with one or multiple aa. phrenicae caudales being the first arteries that extend from aorta abdominalis, these being usually asymmetric. Along with these or separately, a. adrenalis cranialis exits immediately caudally to the abdominal aorta that on the right side may exit a. renalis dextra [8]. Unpaired a. celiaca exits at the level of lumbar vertebra 3 (at the cranial pole of the right kidney) to split, after 10 mm, into a. splenica, left a. gastrica and a. hepatica. A. splenica runs into the splenic hilum, where it splits, prior its entry into the splenic parenchyma, to form 5–8 branches. A. gastrica sinistra runs caudally to the stomach, approaching its small curvature to the right of the oesophagus. Here, it is branching into fully anastomosing visceral and parietal branches that form ramifications on the corresponding surface of the stomach. A. hepatica runs cranially between v. portae and the right side of the papillary tip of the liver (processus papillaris hepatis) into porta hepatis. Before moving from the small curvature of the stomach, it produces a. gastroduodenalis, which then splits to form a. gastroepiploica dextra and a. pancreaticoduodenalis cranialis. The aforementioned trunk runs caudally through the pylorus to the large curvature of the stomach, where its course forms considerable meanders. The branch for the pylorus is called, by Mickwitz [8], a. gastrica dextra. A. pancreaticoduodenalis cranialis runs caudally in the mesoduodenum, where it is producing numerous branches for the pancreas to eventually anastomose with a. pancreaticoduodenalis caudalis. One of the branches stemming from the final branching of the hepatic artery is supplying the caudal portion of the oesophagus through the ascending and descending arterioles (called a. hepatoesophagica by [7]). Another branch exiting aorta abdominalis is a. mesenterica cranialis, this taking place about 3–5 mm caudally behind a. celiaca. The first branch of this is (often doubled) a. colica media that supplies colon transversum and the front segment of colon descendens. It is often anastomosing with a. colica sinistra that exits a. mesenterica caudalis. Another artery to be produced by a. mesenterica cranialis is a. pancreaticoduodenalis caudalis. Shortly after its exiting, it splits into its right branch and left branch. While the left branch is producing branches for the pancreas, splitting near the duodenum to form its cranial part and caudal part, the right branch supplies the ascending duodenum. Both of the branches anastomose with one another as well as the nearby intestinal arteries. Next, a. colic dextra, runs to colon ascendens, producing 16–20 loosely branched aa. jejunales that supply the jejunum and the proximal portion of the ileum. The primary continuation of a. mesenterica cranialis, a. ileocolica that produces a branch of the colon for colon ascendens and the ileal branch for the ileum to approximate, as a. cecalis, the caecum near ostium ileocecale. Here, there is this vessel’s bifurcation and branching along the small curvature of the caecum. In accordance with the location of the kidney, there is another artery exiting aorta abdominalis immediately after a. mesenterica cranialis – a renalis dextra. A. renalis sinistra exits the abdominal aorta more caudally about 5 mm further. A. renalis dextra is running dorsally around v. cava caudalis to direct towards the renal hilum, where it splits into several branches. Before branching, it produces a. adrenalis cranialis (a. adrenalis dextra) [5]. A. renalis sinistra turns slightly caudally to the hilum of the left kidney, before which it is producing a. adrenalis caudalis (a. adrenalis sinistra) [5]. Approximately at the level of the caudal pole of the right kidney, paired a. testicularis or a. ovarica exits the abdominal aorta. Within its course, a. testicularis is producing rami epididymales. A. ovarica runs on each side caudolaterally, producing r. uterinus. About 10–15 mm caudally behind the terminal of a. renalis sinistra, there are a. circumflex ilium profunda sinistra and a. circumflex ilium profunda dextra exiting aorta abdominalis under the right angle to the left and more caudally to the right, respectively. These arteries run dorsally to the urethers to head further to the lateral margin of m. quadratus lumborum, into which they enter. They further supply ventral lumbar muscles and the muscles of the abdominal wall and continue as far as the sacrum level to supply the skin. During its course through the abdominal cavity, aorta abdominalis is producing, dorsally, 5 unpaired lumbar arteries – aa. lumbales, each of these splitting to form its left branch and right branch shortly after exiting. The branches supply paraxial and lumbar muscles (rami musculares), the skin (rami perforantes), and, through foramina intervertebralia, the spinal cord (rami spinales). Just before the caudal bifurcation of the abdominal aorta, before the exit of a. sacralis mediana, aorta abdominalis is producing penultimate unpaired branch – a. mesenterica caudalis that may also be exiting directly in the bifurcation. This artery is directed caudally to split, after about 10 mm, into a cranially directed a. colica sinistra supplying colon descendens, and a. rectalis running caudally to the anus. At the level of the caudal end of the last lumbar vertebra, the abdominal aorta splits into (paired) a. iliaca and unpaired a. sacralis mediana.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


