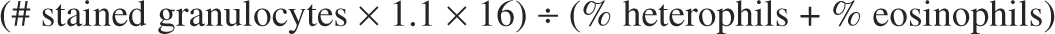9 LABORATORY EVALUATION OF LEUKOCYTES
3 How are leukocytes counted in avian and nonmammalian blood?
The formula for calculating the total leukocyte count (cells/μl) follows:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree