Fig. 17.1
Retroviral mechanism of infection. The retroviral vector infects and integrates the therapeutic gene (clock gene) into the genome of the host cell, and then the cell transcribes the transgene and synthesizes the therapeutic protein
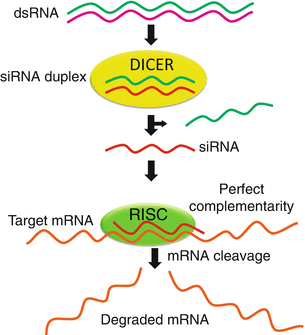
Fig. 17.2
Mechanism of mRNA silencing using small interfering RNA (siRNA). Information coding for a siRNA is transferred using a vector to human cells to degrade specific mRNA sequences to which they are homologous, in this manner silencing the altered gene. Double-stranded RNA (dsRNA) is cleaved by type III RNAse DICER in two smaller fragments of siRNA, sense and antisense strands. Then endonuclease RNA-induced silencing complex (RISC) uses the antisense strand of the siRNA to bind and degrade the corresponding mRNA
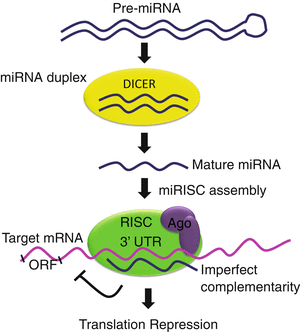
Fig. 17.3
Mechanism of posttranscriptional repression of mRNA by microRNA (miRNA). Information coding for particular miRNAs (small noncoding RNA molecules) is transferred into cells to specifically recognize and precisely regulate mRNA levels, by cleavage of the mRNA strand, and thus promotes repression of mRNA translation. Double-stranded RNA (dsRNA) is processed in the cytoplasm by DICER generating short RNA (22 nucleotides in length). This short fragment is integrated into the RISC complex which contains members of the Argonaute protein family AGO1-4 and becomes an active miRNA. Thereafter miRNAs exert their regulatory effects by binding to imperfect complementary sites within the 3′ untranslated regions (UTRs) of the target mRNA. The formation of the double-stranded RNA, resulting from the binding of the miRNA, leads to translational repression
As of today, most gene therapy clinical trials have been directed to treat cancer, but there are also trials for other neurological diseases (Gene therapy clinical trial worldwide 2014).
17.4.2 Gene Therapy for Clock Genes
Lentiviral and adeno-associated vectors are used as gene transfer systems to produce small interfering RNAs (siRNAs) to diminish or silence the expression of specific genes (Ryther et al. 2005), and they can also be used to express a cDNA coding for proteins or transcription factors like clock genes, lacking in the affected cells (Gene therapy clinical trial worldwide 2014). An important question is whether these methods can be employed in the CNS, without causing secondary damages, due to the vector, due to the method of administration, or by other effects on brain circuitry and functioning. To the best of our knowledge, there are few instances in the field of chronobiology that have used siRNA to silence genes; and in most of these experiments, liposomes were employed as the delivery system. For example, the expression of Bmal1 was downregulated using a siRNA, which reduced the levels of the transcription factor NF-YA (nuclear factor Y A), one of the activators of Bmal1 transcription (Xiao et al. 2013); there is another report using a siRNA to downregulate Rev-erb alpha in pancreatic cells (Vieira et al. 2013), and in luteinizing granulosa cells, Bmal1 was knocked down with an interference RNA (Chen et al. 2013c). Gene delivery is a crucial aspect that needs to be improved for gene therapy to be employed to regulate clock gene expression and activity.
Recent studies have demonstrated that microRNAs (miRNAs) which have key roles in regulating both synaptic plasticity and brain development can repress the expression of genes implicated in cellular mechanisms that regulate mood, cognition, and circadian functions (Hunsberger et al. 2009). For example, in bipolar disorders, the presence of miRNA-134 in circulating blood can be used as a peripheral marker that reflects acute manic episodes and that can be modulated by pharmacological mood stabilizers (Rong et al. 2011). Specifically, Clock and Bmal1 activate the transcription of miR-219, which attenuates the intracellular calcium response to NMDA activation in the SCN (Cheng et al. 2007), and Bmal1 is regulated by miR-142-3p (Shende et al. 2013). Also, miR-24, miR-29a, and miR-30a affect the circadian clock through the regulation of Per1 and Per2 in two ways, first in mRNA stability and second in protein translation (Chen et al. 2013a). In contrast, miR-132 increases calcium influx after depolarization (Cheng et al. 2007). These findings implicate that targeting clock gene-associated miRNAs may be a novel way to regulate clock gene expression.
17.5 Conclusion
There are many questions that need to be resolved in order to develop new treatments, like gene therapy, for neurological and degenerative diseases. As mentioned above, polymorphisms in the CLOCK, BMAL1, PER3, and TIMELESS genes have been associated with mood disorders; is it then possible to develop a gene therapy strategy to regulate the activity of clock genes to normalize their expression and the levels and activity of the proteins associated with circadian rhythm disturbances? Can we associate the influences of some nutrients with the regulation of the clock genes expression? Are the polymorphisms in clock genes a key that can explain the tolerance developed to drugs used to treat these illnesses?
As of now, we have no answers for many of these questions, but this highlights the importance of this field of research since our internal clock needs to function correctly to maintain chronostasis (see Chap. 12).
As previously discussed, there is little experience of gene therapy directed to modify circadian rhythms. Although the literature of experimental strategies based on gene therapy to treat neurological diseases is very abundant, most of the clinical trials of CNS diseases have been directed to treat tumors, particularly gliomas. Furthermore the concept of modifying behavior by gene therapy is highly controversial. An interesting aspect, however, is whether by inducing the recovery of circadian rhythmicity, it would be possible to impact on other diseases, MD, for instance, or whether the use of gene therapy for those diseases would improve circadian rhythmicity.
References
Alenghat T, Meyers K, Mullican SE et al (2008) Nuclear receptor corepressor-histone deacetylase 3 governs circadian metabolic physiology. Nature 456(7224):997–1000PubMedCrossRefPubMedCentral
Capasso C, Garofalo M, Hirvinen M et al (2014) The evolution of adenoviral vectors through genetic and chemical surface modifications. Viruses 6:832–855PubMedCrossRefPubMedCentral
Chen H, Zhao L, Kumazawa M et al (2013c) Down-regulation of core clock gene Bmal1 attenuates expression of progesterone and prostaglandin biosynthesis-related genes in rat luteinizing granulosa cells. Am J Physiol Cell Physiol. doi:10.1152/ajpcell.00008.2013
Cheng H-YM, Papp JW, Varlamova O et al (2007) MicroRNA modulation of circadian clock period and entrainment. Neuron 54(5):813–829PubMedCrossRefPubMedCentral
Cockrell AS, Kafri T (2007) Gene delivery by lentivirus vectors. Mol Biotechnol 36(3):184–204PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


