Chapter 49 Ionophores
SOURCES
Several polyether carboxylic antibiotics (ionophores) are extensively used as anticoccidials in several species and as growth promotants in ruminants. The name ionophore has been used to denote this group of compounds because of their ability to transport ions across biological membranes and down concentration gradients. The ion transport capability is the key to their anticoccidial activities and their antibacterial activities. In ruminants, the antibacterial effects result in changed ruminal microflora and growth promotant effects. Although these compounds have proved very effective for therapeutic use as growth promotants in ruminants and as anticoccidial agents, they also have proved to be toxic in a variety of species. Most cases of small animal poisonings have resulted from inadvertent inclusion of ionophores in dog or cat foods, but pet exposure to feed premixes also occurs.
Several ionophores are in common use and more will likely gain market approval in the near future. Generic and trade names of several marketed ionophores are as follows: laidlomycin propionate (Cattlyst), lasalocid (Avatec, Bovatec), maduramicin (Cygro), monensin (Coban, Rumensin), narasin (Maxiban, Monteban), and salinomycin (Coxistat, Biocox, Saccox).1,2 These ionophorous drugs are marketed in finished feed products, where their concentrations are generally in the part per million range or in milligrams per kilogram quantities. However, they also are marketed at much higher concentrations in the form of premixes or concentrates intended for mixing into finished feeds or for use as a top dressing on feeds.
Because of the variety of concentrations and preparations of products in which ionophores are marketed, there are numerous potential sources of exposure to small animals. Field cases of ionophore poisoning have been reported in which premixes were inadvertently included in cat,3 dog,4,5 and rabbit6 foods. In another case, dog food was stored in the same bin that had previously been used to store a monensin premix.7 In Hungary, rabbits were poisoned with high concentrations of narasin in their pellets.8 Thus ionophore poisonings in small animals can be caused by food mixing errors or storage contamination, or possibly by ingestion of feeds or premixes intended for other species.
TOXIC DOSE
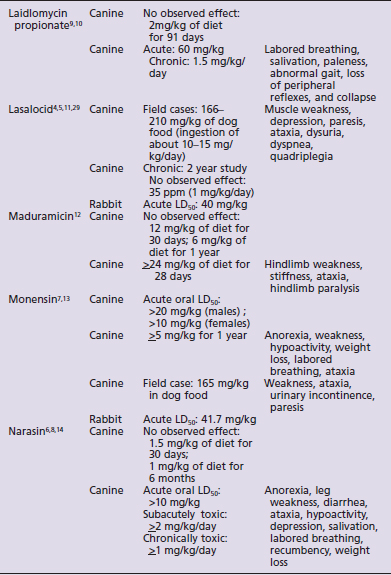
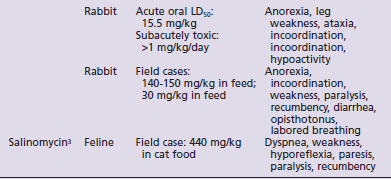
Species susceptibility is quite varied to the toxic effects of the ionophores. The toxicity also is affected by the duration of exposure to ionophores, the daily dose that produces toxic effects being lower with subacute or chronic exposures than the toxic dose for a single acute exposure. Table 49-1 summarizes the literature on the toxicity and clinical signs of several ionophores in small animals. Although toxicity information is available for most ionophores with regard to dogs and rabbits, very little information is available for cats. No toxicity studies and only one clinical report were found for cats.3
TOXICOKINETICS
Although little true kinetic data are available, some toxicokinetic information can be gleaned from the field cases and premarket safety studies. The onset of clinical signs can be either acute or somewhat delayed. With acutely toxic concentrations of ionophores, the onset of clinical signs generally occurs within 6 to 24 hours.4,5,7,14 However, with lower concentrations, clinical signs may not occur for 2 weeks or more.13,14 Thus the onset of toxic effects is dose dependent.
As with the onset of clinical effects, the duration of effects is quite varied. Once ionophore exposure stops, clinical signs may continue for as little as a few days or as long as 3 months.4,5,7,13,14 The duration of clinical signs is thought to correlate with the severity of the clinical signs at the time exposure was terminated. Because ionophores are lipid soluble and clinical severity is dose-related, it is likely that there is either a long terminal elimination phase from the tissue compartments or a longer period of tissue repair in the more severely affected animals.
Ionophores appear to be extensively metabolized and are primarily eliminated in the feces in dogs.10 Less than 2% of a dose of laidlomycin propionate was eliminated in the urine as either the parent drug or its metabolites. Dogs metabolize laidlomycin propionate to at least 11 different metabolites.10 However, it is not known whether the ionophore metabolites retain any of their ionophore transport capability.
MECHANISM OF TOXICITY
Translocation of ions and disruption of ion gradients are responsible for the therapeutic and toxic effects of ionophores.15,16 Translocation of ions across the mitochondria disrupts mitochondrial energy production and causes mitochondrial swelling and fragmentation.17–19 This loss of energy production is at least partially responsible for the cell death and tissue necrosis associated with ionophore poisoning.
The translocation of ions across the plasma membrane by ionophores also inhibits the activity of excitable tissues. The disruption of potassium, hydrogen, calcium, and sodium concentrations in excitable cells alters resting potentials, action potentials, and contractility.20–26 In rat neuronal cell cultures, lasalocid causes neuronal cell damage and death, but spares the nonneuronal cells.27 The alteration in excitable tissues can decrease or halt neurological, cardiac, and skeletal muscle functions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree