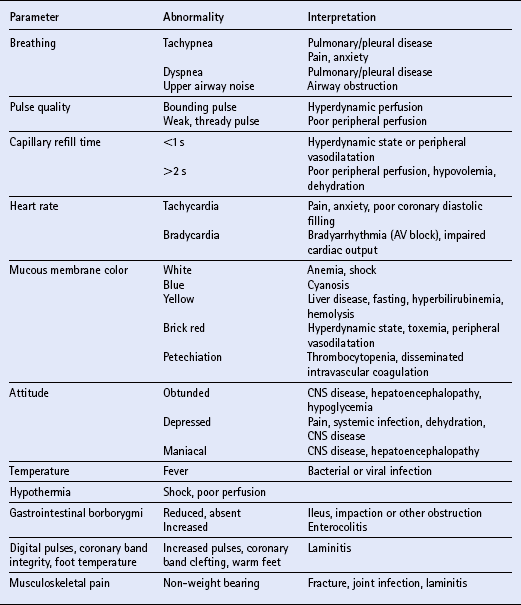
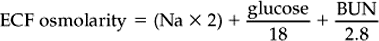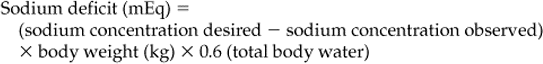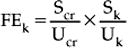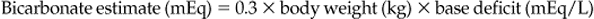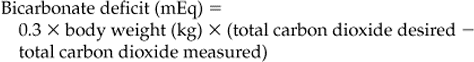Chapter 23 Assessment of hydration status and effective circulating volume Monitoring circulatory status and fluid therapy Assessment of electrolyte disorders Technical considerations in fluid therapy PHARMACOTHERAPY OF THE CRITICALLY ILL NEONATE AND HORSE MANAGEMENT OF ENDOTOXEMIA AND SYSTEMIC INFLAMMATORY RESPONSE SYNDROME PATIENT CARE PROTOCOLS FOR THE CRITICALLY ILL HORSE DESIGN OF AN INTENSIVE CARE UNIT Critical care is an exciting area of equine practice that has undergone rapid advancement and increased specialization in recent years. The increasing pursuit of advanced critical care training and board certification has redefined equine critical care, providing a new focus in the understanding and management of horses requiring intensive care. Creation of a critical care area within an equine hospital allows trained personnel to monitor and care for a number of seriously ill horses most efficiently. The establishment of a nursing station allows for supplies, drugs, delicate instrumentation and monitoring equipment to be centralized. Recumbency complicates the care of many horses suffering from neurologic disorders such as rhinopneumonitis and botulism (q.v.), or musculoskeletal problems such as long bone fractures and laminitis (q.v.). Stall modifications that facilitate care of critically ill patients include wall-mounted medical gases and suction, padded floors and walls, and ceiling hooks for fluid administration. Examples of patient problems that benefit from a critical care setting include the following: hepatoencephalopathy (q.v.), requiring continuous glucose and fluid infusion, sedation, a padded environment and protective head helmet; sepsis (q.v.), causing hypotension and organ dysfunction; renal failure (q.v.), requiring continuous fluid therapy, careful monitoring of fluid administration and urine output, controlled dopamine infusion and diuretic administration; severe corneal ulcers (q.v.), requiring hourly eye medication via a nasolacrimal or subpalpebral lavage system; botulism (q.v.), requiring a padded environment to minimize self-trauma associated with recumbency, frequent patient repositioning to prevent dependent lung atelectasis, continuous fluids and enteral nutrition via nasogastric intubation; severe laminitis (q.v.), requiring padded floors, deep bedding, and supportive care if recumbent; postoperative colic (q.v.), requiring frequent observation for recurrent pain, gastric decompression via nasogastric intubation, and continuous fluid therapy; impaction colic (q.v.), requiring continuous IV fluids and frequent administration of oral fluids and laxatives. Monitoring of any critical patient focuses on frequent evaluation of standard parameters such as those presented in Table 23.1. The primary goals of fluid therapy are to replace and correct these imbalances, to restore effective circulating volume and blood pressure, to provide the body’s maintenance requirements for water and electrolytes, and to account for ongoing losses. Rational fluid therapy requires an understanding of the normal homeostatic mechanisms governing body water, electrolyte and acid-base balance, and the pathophysiologic processes that disrupt their homeostasis. Total body water represents approximately 60–70% of body weight in adult horses and is comprised of the extracellular and intracellular spaces ( Table 23.2). The extracellular compartment includes the intravascular (plasma), interstitial and transcellular fluid (gastrointestinal tract water and lymph). The distribution of water between plasma and interstitial fluid is maintained by differences in colloidal osmotic and hydrostatic pressure, and also depends on the integrity of the endothelium. Water balance is the difference between input and output. This is determined by the intake of water and fluid contained in food, and by the generation of water due to protein, fat and carbohydrate metabolism, versus water loss through urine and feces, respiratory tract and skin. The normal water intake for a 450 kg adult horse is approximately 20–30 L/day, although this can vary depending on the water content of the diet, ambient temperature and activity level. The largest component of water loss is through the urine. Clinical examination is the first, and most important, means of assessment of fluid disorders. The parameters used to evaluate both total body water (hydration status) and effective circulating volume are listed in Box 23.1. Clinical evaluation can be used to provide a rough estimate of the fluid deficit ( Box 23.1). However, these are only guidelines and replacement fluid therapy is best evaluated by serial monitoring of the clinical response. Body weight can be used to determine ongoing fluid losses, however in many clinical situations the initial weight is unknown so it is of little value at the initial evaluation. Fluid deficits will lead to a decrease in urine production, and so measurement of urine production in relation to water intake is useful to monitor fluid therapy. Packed cell volume (PCV) and total plasma protein (TP) are invaluable for assessing fluid deficits and monitoring the response to fluid therapy. They are quickly performed, using a microhematometer and refractometer, respectively. Guidelines for their interpretation are given in Table 23.3. PCV and TP should always be interpreted together. Both rise with simple dehydration. Table 23.3 Interpretation of packed cell volume and total plasma protein concentrations ↑, Increased; ↓, decreased; N, within the reference interval. In horses there is a wide reference range for PCV. Splenic contraction, which occurs under the influence of epinephrine/adrenaline, in association with pain, excitement or other stresses, can increase the PCV markedly but generally does not influence TP. An increased PCV with a normal TP, a relative hypoproteinemia, is also seen in animals that are both dehydrated and hypoproteinemic, a common finding in horses with acute enterocolitis (q.v.). Serum urea and creatinine concentrations ( Table 23.4) are increased in pre-renal, renal and post-renal dysfunctions (q.v.). Urea is produced in the liver as a result of breakdown of ammonia. In horses and other large animals, urea concentrations may be influenced greatly by dietary factors, and as a result creatinine concentration is considered to be a better guide to renal function. Creatinine is produced at a relatively constant rate as a by-product of muscle metabolism (creatine phosphate). It is excreted by glomerular filtration. Decreases in renal blood flow caused by reduction in effective circulating volume produce an increase in serum creatinine concentrations, yielding pre-renal azotemia (q.v.). Restoration of circulating volume should produce a rapid response in serum creatinine in these horses, and if serum creatinine concentrations remain persistently increased after rehydration, the presence of primary renal dysfunction or post-renal causes should be evaluated further. Arterial pressure is measured either directly via a manometer attached to an intra-arterial catheter, or indirectly using Doppler or oscillation techniques. Direct methods are more accurate and have the advantage that the pulse contour can also be assessed, providing information on the inotropic state and the afterload. Indirect methods are non-invasive, and thus are more readily performed in conscious horses ( Table 23.5). While most indirect means of blood pressure measurement provide only systolic pressures, oscillometric techniques, such as the Dinamap Blood Pressure Monitor (Critikon, Tampa, FL, USA), provide systolic, diastolic and mean blood pressures. Correlation of the displayed pulse rate to the actual heart rate is one means of checking the accuracy of the indirect technique, as is averaging repeated measurements. Central venous pressure (CVP; see Table 23.5) is dependent on cardiac output and the venous return. It is the pressure within the intrathoracic vena cava and approximates right atrial pressure. It is influenced by blood volume, vascular tone, heart rate and ventricular contractility. CVP falls in association with hypovolemia and rises if there is an increase in circulating volume. Measurement of CVP is used to assess cardiac function and to monitor fluid therapy and, in particular, to avoid overzealous fluid administration. As such, it can be used as one end point to fluid therapy. Hypotension (q.v.) can be treated with volume support until CVP approaches maximum. Exceeding normal CVP values has the potential to cause pulmonary and tissue edema. CVP is easy to measure, requiring only a simple water manometer, IV catheter and extension tubing. A procedure for the measurement of CVP is described in Box 23.2. It should be noted that the monitoring of trends in CVP over time is crucial, rather than focusing on one value. Pleural and pericardial diseases will also cause increases in CVP (q.v.). Although total protein and albumin concentrations provide indirect information about COP, and are crucial in monitoring albumin dynamics, they are not as well correlated with oncotic pressure in the critically ill animal as compared to healthy horses. In ill patients, direct measurement of COP using a colloid osmometer (Wescor 4420 colloid osmometer, Wescor, Logan, UT, USA) is important. Additionally, total protein concentrations do not measure the oncotic contribution of synthetic colloids such as hetastarch or Hextend. Direct measurement of COP in horses receiving synthetic colloids is the only means of monitoring the oncotic effects of these products. Normal oncotic pressure in adult horses is 20–30 mmHg, and in neonatal foals is 15–23 mmHg. Sodium is the most abundant cation in the extracellular fluid (ECF; see Table 23.4). Sodium is ingested with feed and water intake, and is lost in urine, feces and sweat. It can be regarded as the “skeleton” of the ECF, as it is the principal determinant of the ECF osmolarity, and consequently its volume. Osmolarity can be measured directly with an osmometer, or alternatively calculated using the following formula: where sodium is in mEq/L and glucose and BUN are in mg/dL. The excretion of sodium via the kidney is controlled by the renin–angiotensin–aldosterone system (q.v.). Hyponatremia (q.v.) occurs if there is a reduced intake of sodium, however deficits due to excessive losses are more common. These arise if sodium loss from the gastrointestinal or urinary tract is in excess of water loss, and thus hyponatremia indicates a relative water excess. Box 23.3 lists clinical conditions associated with hyponatremia. Hypernatremia (q.v.) arises when water is lost in excess of electrolytes (see Box 23.3). Both hyponatremia and hypernatremia should be treated with caution; rapid correction of hyponatremia can result in demyelination and central pontine myelinolysis, while cerebral edema can result from overzealous treatment of hypernatremia. Plasma sodium concentrations should be altered slowly, not exceeding 0.5 mEq/h. Hyponatremia can be corrected using sodium-containing fluids such as 0.9% sodium chloride, lactated Ringer’s solution, Plasma-Lyte 148, or Normosol R. Varying formulations of hypertonic saline can also be used depending on the rate of rise. Hypernatremia can be treated with the provision of free water, as with maintenance fluids. These include Plasma-Lyte 56 or Normosol-M, but 5% dextrose in water can also be used. In anorexic horses, potassium can quickly be depleted and diarrhea also frequently results in hypokalemia (see Box 23.3). Severe hypokalemia can be accompanied by muscle weakness and ileus. Management of hypokalemia should include supplementation of fluids with potassium chloride or potassium phosphate. The rate of supplementation should not exceed 0.5 mEq/kg/h. Empirical supplementation of fluids with 10–40 mEq/L of KCl is the usual range utilized to treat or prevent hypokalemia. As hypomagnesemia (q.v.) can be associated with refractory hypokalemia, magnesium concentrations should be monitored in these patients. The causes of hyperkalemia are also listed in Box 23.3. Hyperkalemia must be regarded as an emergency as cardiac arrhythmias (q.v.) frequently occur as a result of the electrical instability of the myocardial cell membrane if serum potassium concentrations rise. Electrocardiographic changes associated with hyperkalemia include spiked T waves, prolonged PR interval, disappearance of P waves, prolongation of the QRS complex, shortening of QT or ST intervals and ventricular dysrhythmias (q.v.). Management of hyperkalemia includes the use of potassium-free fluids, dextrose, insulin and sodium bicarbonate. Dextrose can be added as a 5% solution. Insulin should be utilized only when plasma glucose concentrations can be monitored frequently and may be given as regular insulin (0.01–0.1 U/kg/h). Sodium bicarbonate should be directed by blood gas analysis, but safe amounts can be administered at a dose of 0.5–1 mEq/kg over 30–60 min assuming the patient is not hypoventilating. Calcium supplementation can be used to antagonize the membrane effects of potassium, but will not lower potassium concentrations directly. Enhancement of potassium clearance can be accomplished with exchange resin enemas (polystyrene sulfonate or Kayexalate) and the use of loop or other potassium-excreting diuretics. Chloride is present in high concentrations in the ECF (see Table 23.4). At most sites within the body, chloride tends to follow sodium passively by diffusion within the cell membrane, so that the regulation of chloride concentration in the ECF is directly related to the sodium concentration. In the ECF, chloride concentrations are inversely related to bicarbonate concentrations. Chloride is a strong anion, contributing to strong ion difference (SID). In horses, chloride depletion occurs if there is loss of chloride-rich fluid from the proximal gastrointestinal tract, thus hypochloremia may be associated with esophageal obstruction (q.v.) or with nasogastric reflux due to ileus or proximal gastrointestinal obstruction (q.v.). Hypochloremia is usually accompanied by metabolic alkalosis (see Box 23.3) but may be seen in states of metabolic acidosis during attempts at metabolic compensation through enhanced excretion of urinary chloride. Calcium is an abundant divalent cation throughout the body. Absorption of calcium is via the gastrointestinal tract. It has an integral role in multiple biological processes, including nervous, skeletal, smooth muscle and myocardial function, and blood clotting. It is excreted by the kidney, and bone represents a large calcium reservoir. Calcium homeostasis is regulated by the hormones parathormone and calcitonin (q.v.). Hypocalcemia associated with lactation may produce signs of tetany, particularly in mares exposed to an additional stressor such as transport (see Box 23.3). Mild hypocalcemia is frequently observed with abdominal crises, and it may contribute to postoperative ileus (q.v.) and weakness in some of these horses. Hypercalcemia (see Box 23.3) is usually due to chronic renal failure (q.v.), a biochemical abnormality unique to the horse. Paraneoplastic syndromes (pseudohyperparathyroidism) and vitamin D excess are uncommon causes of hypercalcemia. Hypercalcemia is more difficult to treat, but as a minimum, calcium intake should be reduced by feeding a diet low in calcium; alfalfa should be avoided. The assessment of electrolyte disorders must be based on laboratory evaluation of serum concentrations of electrolytes. Specific electrolyte disorders cannot be predicted simply from the clinical presentation or problem. Serum concentrations of sodium are accurate in assessing sodium deficits or relative excesses, as sodium is an extracellular ion. In contrast, measurement of serum potassium concentration does not reflect the whole body content of potassium, as this is primarily an intracellular ion. Acid-base status further complicates the interpretation of serum potassium concentrations. A decrease in pH of 0.1 units causes an increase in serum potassium concentration of 0.6 mEq/L. Thus, acidosis can mask potassium depletion by increasing the concentration of potassium in the ECF. This is seen commonly with bicarbonate and potassium losses associated with enterocolitis (q.v.). where Scr is serum creatinine concentration, Ucr is urine creatinine, Uk is urine concentration of potassium, and Sk is serum concentration of potassium. Acid–base disorders may be classified as respiratory or metabolic in origin (see Box 23.3), and mixed acid-base disorders are common. Failure of ventilation results in the inability to remove carbon dioxide (respiratory acidosis), while hyperventilation decreases carbon dioxide and causes respiratory alkalosis. It is important to appreciate that acid-base disorders of respiratory origin cannot be alleviated by fluid therapy and require modification of respiratory function. Metabolic acidosis can be the result of excessive production of organic acids (titration) or of loss of bicarbonate (secretion). The titration form of metabolic acidosis occurs when there is an increase in acid anions and bicarbonate is required for buffering. In the horse, lactic acidosis (q.v.) occurs commonly. It is usually associated with hypovolemic, cardiogenic or endotoxic shock (q.v.) and inadequate tissue perfusion. The secretion form of metabolic acidosis occurs when bicarbonate ions are actively lost from the body (see Box 23.3). In some conditions, both secretion and titration of bicarbonate will occur. An arterial blood gas analysis is required for full evaluation of both the respiratory and metabolic components of acid–base balance. Normal values for arterial and venous blood gas analysis are given in Table 23.6. Interpretation of the respiratory component is based on the partial pressure of carbon dioxide in arterial blood ( Table 23.7). Metabolic disorders are manifested by alterations in bicarbonate concentrations and the base deficit. Both arterial and venous blood gas analysis are equally suitable for assessment of the metabolic component of acid–base disorders (see Table 23.6). Table 23.6 Reference intervals for blood gas analysis in horses Table 23.7 Interpretation of blood gas analysis and acid–base status ↑, Increased; ↓, decreased; N, normal. NB: In acid-base disorders, compensation may result in the following changes: Metabolic acidosis: PCO2 decreases 1.2 mmHg for every 1 mEq/L decrease in [HCO3−]. Metabolic alkalosis: PCO2 increases 0.6–1 mmHg for every 1 mEq/L increase in [HCO3−]. aAnion gap = ([sodium]+[potassium])−([bicarbonate]+[chloride]). The anion gap (the difference between cations and measured anions) is used to distinguish titration and secretion forms of metabolic acidosis (see Table 23.7). The anion gap is a reflection of those ions that are not measured, organic acids, phosphates, sulfate and protein. Lactic acidosis increases the anion gap, whereas the anion gap is unchanged when the primary problem is loss of bicarbonate. In that situation, chloride concentrations increase as bicarbonate concentrations fall to maintain electrical equilibrium. An increase in the anion gap with a normal pH and bicarbonate are the hallmarks of mixed metabolic acidosis and alkalosis (see Table 23.6). Without assessment of the anion gap, this abnormality might otherwise go unnoticed as alterations in both pH and bicarbonate are counteracted by the conflicting processes. The ideal crystalloid solution for volume replacement has a composition similar to that of plasma. These include lactated Ringer’s (Hartman’s) solution or similar balanced polyionic solutions such as Plasma-Lyte (Baxter Health Care Corp., Deerfield, IL, USA [available in 5L bags]), Multisol-R (Normosol-R, CEVA) and Isolec (IVEX Ltd, Larne, Northern Ireland, UK [available in 5L bags]) ( Table 23.8). Lactated Ringer’s solution (LRS) contains calcium, whereas Plasma-Lyte and Normosol contain magnesium. Table 23.8 Composition and indications for use of intravenous fluids Iso, isotonic; Hyper, hypertonic. aThese solutions are not designed for maintenance as their sodium concentration is excessive. However, they are commonly used for this purpose, largely for convenience, and are safe provided renal function is normal. Consideration should be given to simultaneous administration of 5% dextrose if they are to be used for maintenance purposes for extensive periods. bNormal saline should not be used for volume replacement, but only to correct specific sodium or chloride deficits, and as an acidifying solution. cHypertonic saline is contraindicated in renal failure and must always be followed with isotonic solutions. Seven per cent sodium chloride is made by dissolving 175g sodium chloride in 2.5L of water. dSerum potassium concentration must be monitored carefully. eIsotonic bicarbonate is made by adding 250 mL of 5% sodium bicarbonate (150 mEq Na: 150 mEq HCO3−) to 750 mL of sterile water. Plasma is a suitable alternative for rapid replacement of circulating volume (see Table 23.8). However, in the volumes required it is often prohibitively expensive to be used for colloid support alone. Under these circumstances, plasma should be used in combination with synthetic colloids such as hetastarch. Fluid overload produces pulmonary edema, thus the respiratory rate and effort and the quality of the lung sounds should be evaluated frequently during the rapid IV administration of large volumes. CVP is the most objective guide to the rate of fluid administration, but examination of the height of the jugular pulse also gives a crude estimation of rising CVP. CVP can be easily measured in both horses and foals, through the use of central lines and a water manometer as described in Box 23.2. Optimization of the CVP can be used as a fluid administration end-point goal. Once near maximum CVP (10 cmH2O in a neonate; 12–15 cmH2O in an adult) is achieved using fluid loading, rates of administration should be decreased to avoid risks of edema formation. Hypertonic saline (7%) is a valuable alternative for the emergency resuscitation of horses with hemorrhagic, hypovolemic or endotoxic shock (see Table 23.8). Administration of hypertonic solutions increases the effective circulating volume, cardiac output and blood pressure. The precise mechanisms by which it is effective are still unclear. A number of processes may be involved, including a vagally mediated reflex, redistribution of blood from peripheral to central vascular beds, increasing cardiac contractility, and relocation of fluid from the interstitium and ICF to the intravascular space. or, if total carbon dioxide (TCO2) is measured, as on a serum biochemistry profile: Sodium bicarbonate is available as 5% and 8.4% solutions, both of which are hypertonic and should be diluted before use. An isotonic solution of bicarbonate can be made by dilution with sterile water to a concentration of 150 mEq/L of sodium and of bicarbonate (1.3% sodium bicarbonate) (see Table 23.8). Bicarbonate should not be added to calcium-containing solutions as precipitates are formed. Metabolic alkalosis is uncommon in horses. It is seen as a result of loss of hydrogen and chloride ions in nasogastric reflux (q.v.), often accompanied by hypochloremia and hypokalemia. Horses with heavy sweating, such as those in endurance rides, can also develop alkalosis. Therapy is aimed at replacement of chloride, and promotion of excretion of bicarbonate via the kidney by restoration of body water. Normal saline, supplemented with potassium, is the fluid of choice for the treatment of metabolic alkalosis (see Table 23.8). Circulating volume deficits should be addressed. Supplementation with magnesium can also aid in treating refractory alkaloses. Hyponatremia rarely requires specific therapy, however normal saline can be used to increase serum concentrations of sodium. Hyponatremia should be corrected slowly to prevent rapid dehydration of the central nervous system (CNS) thereby leading to central pontine dysmyelinolysis and demyelination (q.v.).
Intensive care medicine
INTRODUCTION
FLUID THERAPY
INTRODUCTION
BODY WATER BALANCE
ASSESSMENT OF HYDRATION STATUS AND EFFECTIVE CIRCULATING VOLUME
Clinical examination
MONITORING CIRCULATORY STATUS AND FLUID THERAPY
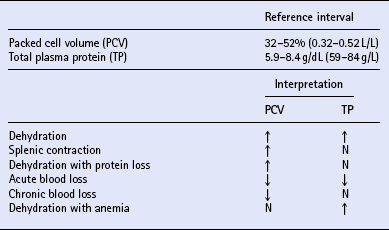
Packed cell volume and total plasma protein
Serum urea and creatinine
Arterial blood pressure
Central venous pressure
Colloid osmotic pressure
ELECTROLYTE BALANCE
Sodium
Potassium
Chloride
Calcium
ASSESSMENT OF ELECTROLYTE DISORDERS
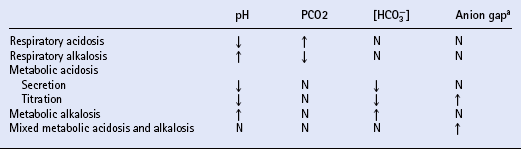
ACID–BASE BALANCE
Assessment of acid–base balance
Arterial
Venous
pH
7.347–7.475
7.345–7.433
PO2 (torr or mmHg)
80–112
37–56
PCO2 (torr or mmHg)
36–46
38–48
HCO3− (mEq/L or mmol/L)
22–29
22–29
Base excess (mEq/L or mmol/L)
−1.7 to + 3.9
−2.7 to + 4.1
REPLACEMENT THERAPY
Crystalloids
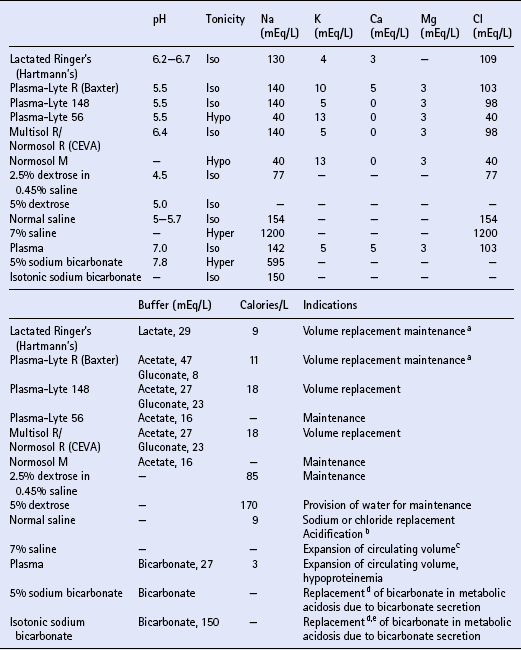
Colloids
METABOLIC ACIDOSIS
METABOLIC ALKALOSIS
Sodium
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Intensive care medicine
Only gold members can continue reading. Log In or Register to continue