Chapter 27. Inherited Disorders of Nutrient Metabolism
Clinical disease can occur in some companion animals as a result of the inability to absorb, assimilate, or metabolize specific nutrients. In some cases, breed predispositions can be found, and the disorder appears to have a genetic basis. Five specific examples in dogs involve lipid metabolism, purine metabolism, and the nutrients vitamin B 12, copper, and zinc. Although inherited disorders of metabolism are less well documented in cats, a familial hyperlipidemia has been reported in this species (Table 27-1).
| D isorder | B reeds affected | T reatment |
|---|---|---|
| Malabsorption of vitamin B 12 | Giant Schnauzer | Intramuscular injections of B 12 |
| Copper-storage disease | Doberman Pinscher | Copper-restricted diet, zinc acetate supplementation |
| Bedlington Terrier | ||
| West Highland White Terrier | ||
| Cocker Spaniel | ||
| Lethal acrodermatitis | Bull Terrier | None |
| Zinc malabsorption | Siberian Husky | Zinc supplementation |
| Alaskan Malamute | ||
| Great Dane | ||
| Doberman Pinscher | ||
| Hyperlipidemia | Miniature Schnauzers, cats | Restricted-fat, restricted-calorie diet |
| Abnormal purine metabolism | Dalmatians | Reduced-purine diet, production of alkaline urine, adequate hydration, allopurinol |
MALABSORPTION OF VITAMIN B 12
Vitamin B 12 (cobalamin) is required by the body as a coenzyme for several metabolic reactions and for normal deoxyribonucleic acid (DNA) synthesis and erythropoiesis. Vitamin B 12 deficiency results in macrocytic anemia and neurological impairment. Absorption of B 12 from the diet requires the presence of a compound called intrinsic factor (IF). In the dog, IF is produced by the gastric mucosa and the pancreas and binds to cobalamin as it passes through the gastrointestinal tract. 1 The IF-B 12 complex attaches to specific receptor sites on cells lining the intestinal mucosa and is absorbed into the body. Without the presence of IF, cobalamin absorption is severely impaired.
Like other species, dogs require very small amounts of dietary vitamin B 12 because of the body’s ability to store adequate amounts of B 12 in the liver for long periods of time. In addition, efficient reabsorption of excreted vitamin B 12 through the enterohepatic circulation results in efficient conservation of this nutrient. As a result, naturally occurring deficiencies of vitamin B 12 are not common in the canine species.
Inherited vitamin B 12 malabsorption was first identified in Giant Schnauzers. Analysis of pedigrees and a series of breeding studies demonstrated a simple autosomal recessive mode of inheritance in this breed. 2 The disorder has since been reported in Border Collies, Australian Shepherds, Beagles, Shar-Peis, and cats. 3.4.5. and 6. Studies of a human condition, Imerslund-Grasbeck syndrome (I-GS), characterized by B 12 malabsorption and juvenile-onset megaloblastic anemia, revealed a similar mode of action to that of Giant Schnauzers and Australian Shepherds with B 12 malabsorption. 7 Studies revealed that B 12 malabsorption in dogs is most often due to mutation of the amnionless gene, whereas in humans I-GS is most often related to mutation of the cubilin gene, both of which are essential for proper IF function. 8
Clinical signs develop when puppies are between 6 weeks and 5 months of age, and include lethargy, failure to thrive, loss of appetite, neutropenia (decreased white blood cell count), and nonregenerative anemia. Vitamin B 12 deficiency can be diagnosed in affected puppies as early as 2 weeks of age by comparing their serum cobalamin concentration with that of normal littermates. 9 Other signs that have been reported include elevated plasma ammonia levels and hyperammonemic encephalopathy. 3.6. and 10. However, the underlying cause of these signs is not completely understood. 9
Vitamin B 12 malabsorption diagnosis can be confirmed through analysis of serum B 12 levels, response to parenteral administration of the vitamin, the presence of elevated levels of methylmalonic acid in the urine, and genetic testing. For example, DNA genetic testing of Giant Schnauzers and Australian Shepherds is available at the University of Pennsylvania Metabolic Genetic Screening Laboratory. 10 Elevated urinary levels of methylmalonic acid can confirm the diagnosis because it is excreted only when the normal metabolism of certain amino acids, fatty acids, and cholesterol is blocked because of the lack of a necessary B 12-containing coenzyme. Normally, dogs excrete less than 10 milligrams (mg) of methylmalonic acid per gram (g) of creatinine in the urine. Giant Schnauzers with vitamin B 12 malabsorption excrete between 4000 and 6000 mg/g of creatinine. 11
Tests have shown that the intestinal absorption of nutrients in affected dogs is normal, with the exception of vitamin B 12. Moreover, oral administration of vitamin B 12, with or without IF, is not effective in resolving clinical signs or in raising serum B 12 levels. These results and immunoelectron microscopy studies of ileal morphology indicate the defect may be located at the level of the cell receptor in the small intestine. Specifically, affected dogs lack a receptor for the IF-cobalamin complex in their brush border microvilli. 2 Long-term treatment of this disorder involves the regular administration of intramuscular injections of vitamin B 12. This injection bypasses the intestine and provides tissues with the necessary vitamin. Complete resolution of clinical signs has been reported with a dosage as low as 1 mg every 4 to 5 months. In other cases, a weekly dosage of 0.5 mg has been used. 10
COPPER-STORAGE DISEASE
Copper is an essential micronutrient, needed for iron absorption and transport, hemoglobin formation, electron transport proteins, various antioxidants, and normal functioning of the cytochrome oxidase enzyme system (see Section 1, p. 41 and Section 2, pp. 113-114). 12 The normal metabolism of copper in the body involves the passage of excess copper through the liver and excretion in bile. Disorders that affect bile excretion often result in an accumulation of copper in the liver, sometimes to toxic levels. In these cases, copper toxicosis in the liver is a secondary disorder that develops as an effect of the primary liver disease. 13 Hepatopathy (acute hepatic necrosis, subacute hepatitis, chronic hepatitis, and cirrhosis) associated with increased liver copper concentration has been noted in many dog breeds including Cocker Spaniels, Dalmatians, German Shepherd Dogs, Keeshonds, Kerry Blue Terriers, Labrador Retrievers, Old English Sheepdogs, Poodles, Samoyeds, and rarely, even in cats. 14.15.16.17. and 18.
A primary, hepatic copper-storage disease, also known as copper toxicosis, has been conclusively identified in the Bedlington Terrier and may also occur in certain other breeds of dogs. Inherited canine copper-storage disease involves the impaired removal of copper from the liver, resulting in accumulation of the mineral as the dog ages. Its development is independent of diet and eventually will cause chronic, degenerative liver disease. The mode of inheritance in Bedlington Terriers is a simple, autosomal recessive gene that shows no sex predilection. 19.20.21. and 22. A variation of this disorder occurs in West Highland White and Skye Terriers. 21. and 23. The mode of inheritance in these breeds is not fully understood. The concentration of copper in the liver does not reach levels that are as high nor do the copper levels consistently increase with age as observed in Bedlington Terriers. 15. and 24. Other breeds that have been reported to be at risk for familial diseases of copper metabolism include Dalmatians, Cocker Spaniels, Labrador Retrievers, and Doberman Pinschers. 21. and 25. Because only female Doberman Pinschers appear to be affected, a sex-linked mode of inheritance is suspected in this breed. 26 In addition, affected Dobermans show reduced copper excretion and increased oxidative stress, which suggests this is a new variant of primary copper toxicosis. 27
Inherited copper-storage disease in Bedlington Terriers is caused by impaired removal of copper from the liver, resulting in accumulation of the mineral as the dog ages. This eventually results in chronic, degenerative liver disease in most animals. Normal liver copper concentration in dogs ranges from 200 to 400 parts per million (ppm) of dry weight (dw), and this level remains constant throughout life. 28 Bedlington Terriers with copper-storage disease begin to accumulate the mineral shortly after birth. Biopsies of the livers of affected puppies show increased copper in the hepatocytes as early as 5½ months of age. 29 During the first few months of life, while copper is accumulating, there is no liver damage, and serum levels of liver enzymes remain within normal range. But when hepatic copper reaches a toxic level of approximately 2000 ppm, centrolobular hepatitis with concomitant elevation of liver enzymes develops. Individual dogs vary significantly regarding the age at which toxic levels are reached and in their susceptibility to clinical signs. Even after toxicity occurs, levels continue to accumulate, sometimes reaching as high as 10,000 ppm dw. 13
The identification of a DNA marker for the copper toxicosis locus in Bedlington Terriers led to the ability to reliably diagnose individual cases and identify pedigrees and lineages in which the gene for this disorder is prevalent. 30. and 31. However, recently this diagnostic method has been complicated by the discovery of diverse haplotypes that lead to false negatives in some Bedlington terriers with copper toxicosis. 32. and 33. Therefore serum chemistry profiles continue to be useful as a preliminary screening tool for the onset of liver disease in young dogs. Liver biopsies should be considered if elevated levels of the liver enzyme alanine amino transaminase are observed. Clinical signs of disease do not usually manifest until the dog is between 4 and 8 years old, although some may show signs as early as 1 year or as late as 11 years of age. 21 Widespread liver necrosis and postnecrotic cirrhosis begin to cause clinical signs that are associated with liver disease. Lethargy, anorexia, vomiting, abdominal pain, and occasionally ascites and icterus are observed. Some dogs suffer acute tubular necrosis in the kidneys and show polyuria and polydipsia in addition to signs of liver disease. 21 Acute episodes of liver necrosis may cause sudden death in a small number of affected dogs.
Treatment involves lifelong feeding of a copper-restricted diet and the administration of medications that either decrease intestinal absorption or increase urinary excretion of copper. 21. and 34. Two chelating agents, penicillamine and trientine, have been used in dogs with copper-storage disease and act by increasing urinary excretion of copper. 35. and 36. However, despite the reported use of these drugs, controlled efficacy and treatment regimen studies have not been conducted in dogs, and penicillamine may be toxic in some animals. 21. and 34. Zinc acetate, which functions to block the intestinal absorption of copper, may be the treatment of choice for dogs with copper-storage disease. 34. and 37. Results of a study that examined the efficacy of zinc acetate in the treatment of copper-storage disease in Bedlington Terriers and West Highland White Terriers found that administration at dosages that resulted in plasma zinc concentrations of 200 to 500 micrograms (μg)/deciliter (dl) suppressed hepatic inflammatory disease and reduced hepatic copper concentrations. It appeared that hepatic function could be restored by the long-term administration of zinc acetate in affected dogs. The administration of 100 mg of zinc acetate twice daily is recommended for the first 3 months of treatment. After this period, the dosage can be reduced to 50 mg twice daily. For maximum effectiveness, the zinc should not be administered with the dog’s food. Plasma zinc concentrations should be measured every 2 to 3 months to confirm that the level has increased appropriately and that the concentration does not exceed 1000 μg/dl. Affected dogs require lifelong therapy and their copper status must be monitored closely to guard against the potential for copper deficiency.
ZINC MALABSORPTION
Although zinc deficiency and zinc-responsive dermatosis can be caused by feeding an imbalanced diet, another potential cause involves an inherited predisposition for impaired zinc absorption. Several breeds of dogs appear to be affected by zinc malabsorption, and varying levels of severity for this disorder have been reported. The most severe zinc-related disorder is lethal acrodermatitis in Bull Terriers. This genetic disease is inherited as an autosomal recessive gene and results in an inability to absorb dietary zinc, even when high levels of the mineral are added to the diet. 38 At birth, affected puppies show lighter pigmentation than is normal for the breed. Their growth is stunted, and severe skin lesions develop by 6 to 10 weeks of age. 39 Cell-mediated immunodeficiency, acquired T-cell deficiency, poor responsiveness of T lymphocytes to mitogen stimulation, and selective deficiency of immunoglobulin A (IgA) also occurs. 40. and 41. The immunodeficiency results in increased susceptibility to pyoderma and multiple infections throughout the body. There is also some evidence that the behavioral disorders of tail-chasing and idiopathic aggression in this breed may be related to zinc malabsorption. 42 This disorder is invariably fatal and has a median survival age of only 7 months. Nearly all affected dogs die before 15 months of age and there is currently no reliable test that can identify carriers of this disease. 38
Less severe zinc-responsive disorders occur in Alaskan Malamutes, Siberian Huskies (Figure 27-1), and, occasionally, Great Danes and Doberman Pinschers. 43.44.45.46. and 47. Research has shown that Alaskan Malamutes afflicted with inherited chondrodysplastic dwarfism have an impaired ability to absorb intestinal zinc. 47 Dwarfism in this breed has a simple autosomal recessive inheritance, and zinc malabsorption appears to be a component of this disorder. However, impaired zinc absorption has also been described in Malamutes that are not afflicted with chondrodysplasia. 43 The mode of inheritance of the zinc-responsive dermatoses is currently unknown. The onset of this syndrome usually occurs at puberty, and some dogs show signs only during times of physiological stress, such as pregnancy or exposure to weather extremes. Dermatological signs include crusting, scaling, and underlying suppuration around the face, elbows, scrotum, prepuce, and vulva. In chronic cases, hyperpigmentation of the affected skin surface is seen. The dogs are usually nonpruritic until the lesions have become extensively crusted. Mild to moderate weight loss and a dull, dry coat are also observed. Histopathological examinations of skin biopsies show diffuse parakeratotic hyperkeratosis.
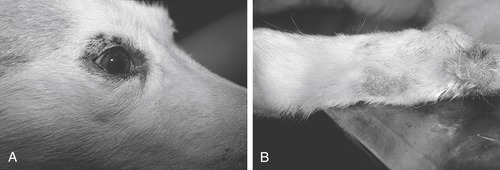 |
| Figure 27-1 (Courtesy Candace Sousa, DVM, Animal Dermatology Clinic, Sacramento, Calif.) |
Another zinc-responsive disorder occurs in rapidly growing puppies of many breeds including Beagles, German Shorthaired Pointers, Great Danes, Standard Poodles, and many others. 44 The severity of signs is highly variable within a litter. Some puppies appear nearly normal and others are depressed, anorexic, and emaciated. Hyperkeratotic plaques may occur with thickened, fissured foot pads and planum nasale. In both of these syndromes, oral supplementation with zinc results in rapid resolution of the skin lesions. However, only Malamutes usually require supplementation throughout life to prevent a recurrence of clinical signs. A therapeutic dose of 10 mg/kilogram (kg)/day of zinc sulfate (ZnSO 4) usually suffices. Large dogs are often given 100 to 200 mg of ZnSO 4 twice daily. 44 ZnSO 4 can cause emesis in some dogs, but this can be prevented in most cases by giving the mineral with the dog’s food. In a small proportion of cases, supplementation is necessary only during periods of stress.
DISORDERS OF LIPID METABOLISM
The term hyperlipidemia is sometimes used interchangeably with hyperlipoproteinemia when referring to elevated levels of triglycerides and/or cholesterol in animals that have been fasted for at least 12 hours. 48 However, the term hyperlipoproteinemia specifically refers to excessive circulating lipoproteins. Hypercholesterolemia describes excessive circulating cholesterol, while hypertriglyceridemia refers to elevated concentrations of triglycerides, either of which may occur alone or in combination with hyperlipoproteinemia. 49
Most of the cases of hyperlipidemia seen in companion animals occur secondary to another underlying disorder that affects lipid metabolism. Diseases that may cause secondary hyperlipidemia include diabetes mellitus, hypothyroidism, pancreatitis, nephrotic syndrome, hyperadrenocorticism, cholestasis, hypercholesterolemia, and liver disease. 50.51.52.53. and 54. In addition, certain medications such as glucocorticoids and immunosuppressant drugs may cause transient increases in blood lipid levels. Familial, or primary, hyperlipidemia refers to cases in which a heritable basis for hyperlipidemia can be found. Two well-documented inherited disorders of lipid metabolism occur in dogs and cats: hyperlipidemia in Miniature Schnauzers and lipoprotein lipase (LPL) deficiency in cats. In addition, hypercholesterolemia has been reported in Miniature Schnauzers with hyperlipidemia, in Briards in the United Kingdom, in Shetland Sheepdogs in Japan, and in a family of Rough Collies. 53.54.55. and 56.
Lipoprotein Metabolism
A basic understanding of the mechanisms of lipid transport in the blood is necessary for an examination of hyperlipidemia. Because lipids are insoluble in water, transport in the blood requires complexing with more soluble molecules such as proteins and phospholipids. Free fatty acids (FFAs) are carried in the bloodstream by albumin, a serum protein. Triglycerides and cholesterol esters are carried by lipoproteins, which are spherical macromolecular complexes made up of a lipid core surrounded by a thin outer membrane. Apoproteins, the proteins that are present in the lipoprotein’s outer membrane, are recognition sites for target tissues and act as enzyme cofactors in lipid metabolism reactions. 57
Lipoproteins can be categorized according to their lipid components and resultant aqueous densities. Like humans, dogs have four major classes of lipoproteins, each of which has a principal lipid component and one or more transport functions. 51 Chylomicrons are synthesized in response to the absorption of fat from the intestine and function in the transport of dietary triglyceride to extrahepatic tissues and cholesterol to the liver. Chylomicrons appear in the blood approximately 2 hours postprandially, causing a transient increase in plasma triglyceride concentration. When they are delivered to tissues, the triglycerides are hydrolyzed to fatty acids and glycerol by the enzyme LPL. The second category of lipoproteins, called very–low-density lipoproteins (VLDLs), transport endogenous triglycerides from the liver to extrahepatic tissues for use as an energy source or for storage in adipose tissue. In contrast to chylomicrons, VLDLs are produced continuously, so that in the fasting state, VLDLs are the main carriers of endogenously produced triglyceride. Low-density lipoproteins (LDLs) transport cholesterol from the liver to extrahepatic tissues for incorporation into cell membranes and for steroid hormone synthesis. Finally, the high-density lipoproteins (HDLs) also transport cholesterol, but they are responsible for moving excess cholesterol out of extrahepatic cells back to the liver for excretion in bile, a process called “reverse cholesterol transport.”
Postprandial hyperlipidemia is a natural occurrence that reflects a transient rise in chylomicrons; in dogs it normally resolves within 6 to 10 hours following consumption of a meal. 58. and 59. Hyperlipidemia that persists for 12 hours or more after food is withheld warrants investigation. 51 In dogs, fasting serum triglyceride concentrations greater than 150 mg/dl and/or total cholesterol concentration greater than 300 mg/dl are considered abnormally high. In cats, fasting triglyceride concentrations of greater than 100 mg/dl and/or cholesterol concentrations greater than 200 mg/dl should be investigated. 60
A number of health problems may be caused by persistent hyperlipidemia in companion animals. Hypertriglyceridemia, especially when severe, is associated with abdominal pain, vomiting, diarrhea, anorexia, seizures, hepatomegaly, and the abnormal deposition of lipid in certain tissues. 58.60. and 61. Like some hereditary hypertriglyceridemias in humans, elevated triglyceride levels in dogs and cats may also increase the risk for development of acute pancreatitis. 57. and 62. Hypercholesterolemia is not common in dogs and cats, but has been reported in Shetland Sheepdogs, Beagles, Briards, Collies, and Miniature Schnauzers. 53.54.55.62. and 63. It is generally not associated with as many health risks as is hypertriglyceridemia. Corneal lipid depositions have been reported in dogs with hyperlipidemia and may be the result of elevated blood cholesterol. 64. and 65. For example, a family of Collies was found to have familial idiopathic hypercholesterolemia, and most of the dogs developed corneal lipidosis. 56 In contrast to humans, dogs and cats rarely develop atherosclerosis in response to hypercholesterolemia, and when atherosclerosis is seen, it is usually the result of congenital or spontaneous hypothyroidism. 66
Hyperlipidemia in Miniature Schnauzers
Hyperlipidemia in Miniature Schnauzers is a well-documented familial disorder. 62. and 67. It is reported that many clinically normal dogs of this breed are found to have persistent fasting hyperlipidemia during routine veterinary examinations. 62 There is no sex predilection, and the disorder is usually first seen in Schnauzers that are older than 4 years of age. The hyperlipidemia is associated with elevated triglycerides and is typically characterized by chylomicron excess. Serum cholesterol levels are either normal or slightly increased. 57 A recent investigation of the prevalence of hypertriglyceridemia in Miniature Schnauzers found that nearly a third (32.8%) had triglyceride concentrations that were higher than the reference range for healthy dogs. Only 5.4% of control dogs from the general population have hyperlipidemia, which does suggest a genetic predisposition in this breed. 68 Increased serum lipase and amylase activities have been recognized in hyperlipidemic Miniature Schnauzers that present with acute pancreatitis. In these cases, the pancreatitis is believed to be caused by the hypertriglyceridemia.
Affected dogs are either asymptomatic or have recurrent episodes of abdominal pain or distress, vomiting, and/or diarrhea. Seizures have also been associated with persistent hyperlipidemia in this breed. 67 Owners may report that episodes of abdominal distress last several days, followed by spontaneous recovery. In many cases, the clinical signs and history are similar to those of dogs with acute pancreatitis, but radiographic and laboratory evidence does not often support this diagnosis. This syndrome has been termed “pseudopancreatitis” by one investigator. 62 Hyperlipidemia in Miniature Schnauzers is believed to be hereditary because of the high breed predisposition and because most affected Miniature Schnauzers lack evidence of diseases that cause secondary hyperlipidemia. 57
The underlying cause of primary hyperlipidemia in Miniature Schnauzers is not known, but is characterized by excessive VLDL particles with or without concurrent chylomicronemia, and mild hypercholesterolemia. 57 LPL activity was decreased in lipemic Miniature Schnauzers compared to LPL activity in nonlipemic Miniature Schnauzers and in other breeds. 69 It is theorized that either a familial deficiency of the enzyme LPL or the absence of an apoprotein that functions to activate LPL may be responsible. The enzyme LPL is located in capillary and endothelial tissue and hydrolyzes the triglycerides that are transported by chylomicrons and VLDLs for transport into cells. A defect in the synthesis or activity of this enzyme prevents the delivery of dietary triglycerides to tissues and leads to the retention of chylomicrons and impaired VLDL metabolism. The absence of an important apoprotein called apolipoprotein C-II (apo C-II) would have a similar effect. Apo C-II is normally a component of chylomicrons and VLDL and is a cofactor for LPL. In humans, individuals with an apo C-II deficiency have clinical symptoms similar to individuals with LPL deficiency. 70. and 71.
Hyperlipidemia in Miniature Schnauzers is a well-documented familial disorder. Although not all dogs show clinical signs, more than 30% of the breed may be affected. Because signs mimic those of acute pancreatitis, the disease has been termed “pseudopancreatitis.”
Feline Lipoprotein Lipase Deficiency
A well-recognized inherited deficiency of LPL in cats causes hyperchylomicronemia and has been shown to be inherited as an autosomal recessive trait. 72.73.74. and 75. The hyperlipidemia is caused by markedly elevated fasting triglyceride concentrations as a result of increased chylomicrons and, to a lesser extent, VLDLs. 76 Clinical signs may or may not be present, and the severity of clinical disease is not well correlated with the degree of hyperlipidemia. The age of onset of clinical signs varies from as young as 3 weeks to middle age. 76 Cats homozygous for LPL deficiency have a lower percentage of body fat compared to heterozygous carriers or normal cats, but lean body mass is not influenced. 77 Kittens suckling queens homozygous for LPL deficiency are at a distinct disadvantage and often must be hand reared or fostered. 78
When cats present with clinical disease, the most common signs include the development of subcutaneous xanthomas (lipid deposits) and lipemia retinalis. The xanthomas occur most often in areas of the body where trauma caused damage to capillaries, leading to extravasation of lipids. 79 Variable peripheral neuropathies are seen in some cases. The signs of nerve damage develop slowly and are characterized by the loss of conscious proprioception and motor function, with retention of sensation of pain. These neuropathies are thought to be caused by compression of nerves by lipid granulomata at sites of trauma. 57 A study of a family of cats reported that LPL-deficient cats produced an abnormal LPL protein that failed to bind normally to vascular endothelium, rendering it inactive. 74 These results support the theory that an inherited disorder of lipid metabolism involving a deficiency of active LPL occurs in the cat.
A well-recognized inherited deficiency of lipoprotein lipase in cats causes hyperlipidemia and appears to be the result of an autosomal recessive trait. The most common clinical signs include subcutaneous xanthomas and lipemia retinalis. Some cats develop peripheral neuropathies that are slowly progressive and characterized by the loss of conscious proprioception and motor function.
Diagnosis of Primary Hyperlipidemias
The diagnosis of primary hyperlipidemia is one of exclusion, as all causes of secondary hyperlipidemia must be ruled out first. Primary hyperlipidemia is seen most frequently as a familial disorder in Miniature Schnauzers and Beagles, although other breeds may be affected. 80 The pet’s breed, family lineage, age, and clinical history can be used to support a diagnosis. A 12- to 18-hour fasting blood sample should be taken and cholesterol and triglyceride concentrations should be measured. 48. and 81. If there is a history of recurrent abdominal pain, vomiting, or diarrhea, serum amylase and lipase activities should be measured to monitor pancreatic pathology.
Quantification of the plasma concentrations of each lipoprotein class may assist in the differential diagnosis of hyperlipidemia. An estimate of the lipoprotein pattern can be obtained through electrophoresis, but major differences exist between dog, cat, and human plasma lipoproteins in circulation. Dogs and cats have HDL predominance and are more resistant to LDL and cholesterol elevations than humans. 82 A precipitation technique for canine plasma lipoprotein quantitation has been published, but has not been widely adapted for routine veterinary clinical use. 83 Some human commercial laboratories offer precipitation and electrophoresis studies to analyze and interpret dog and cat sera, but this approach should only be used if staff are specifically trained to interpret electrophoresis scans of canine lipoproteins. 82. and 49. Even with expert interpretation, electrophoretic separation of lipoproteins is not a disease-specific technique and cannot always differentiate between the functional classes of elevated lipoproteins. 58 Laboratory techniques that can accurately identify lipoproteins that are not adequately differentiated by electrophoresis are not used by most diagnostic laboratories, and so samples must be referred to research laboratories. 57
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


