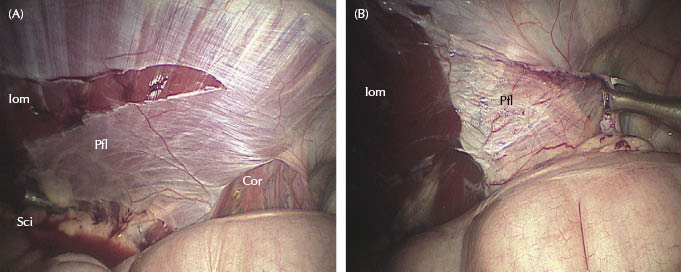
Figures 27.3 (A,B) Left vaginal ring. Laparoscopic view in dorsal recumbency showing the dissection of the peritoneal flap. Sci, scissors; Pfl, peritoneal flap; Cor, testicular cord; Iom, internal oblique muscle after peritoneal flap removal.
For this procedure, laparoscopic scissors are introduced on the ipsilateral side and Babcock forceps (Optomed) on the contralateral side. The flap is inverted and drawn dorsomedially to cover the vaginal ring (Figure 27.3B). It is then fixed laterally and, if necessary, medially to the parietal wall, 2 cm dorsal to the ring. Any dead space around the mesorchium should be covered by the flap and fixed to the parietal wall. Two simple intracorporeal sutures are performed using 3-metric braided lactomer 901 staples (Polysorb, Covidien, Magny-Vernois, Lure Cedex, France) or laparoscopic helicoidal staples (ProTack, Covidien) (Figure 27.4). With the latter technique, it is usually necessary to create one more instrument portal on each side, between the laparoscope and the first instrument portal, to position the stapling device perfectly perpendicular to the tissue. A 5-mm trocar cannula can be used. Five to eight staples are necessary to fix the flap, and the surgeon should apply at least one staple close to the mesorchium, craniolaterally and sometimes caudomedially, in order to optimize the obturation of the inguinal canal. Pushing the mesorchium medially and laterally using a Babcock forceps may help. Intracorporeal suturing enables stronger, more secure attachment of the peritoneal flap, but it is technically more challenging and takes longer compared with staples. If used, it is important for the surgeon to introduce the needle through the 10-mm cannula by grasping the thread about 1 cm from the base of the needle. The assistant should place a finger on the entry of the cannula when the suture is passed in order to prevent any loss of distension gas. Flap security is tested manually before closure. If necessary, an additional suture or staple can be added. Incisions are closed in one layer using one to two simple interrupted skin sutures.
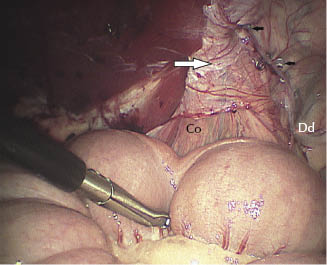
Figure 27.4 Left vaginal ring. Fixation of the peritoneal flap using helicoidal staples. Note the position of the flap (large white arrow) and the helicoidal staples (black arrows). Co, testicular cord; Dd, deferent duct.
Postoperative Management
After recovery, parenteral antibiotics are continued for 24 hours and anti-inflammatory treatment for 3 days. The horse is discharged from the hospital 48 hours post-op. After 8 days of strict stall rest, the horse can be hand-walked twice daily for 2 weeks before returning progressively to training. Closure of the vaginal ring can be assessed after 1 month either by rectal palpation or, when possible, by direct viewing using a standing laparoscopy.
Conclusion
Procedure-Related Complications
Complications are first related to laparoscopy under general anesthesia, especially in the Trendelenburg position. More specific complications to this technique may include puncture of one of the superficial epigastric vessels at the site of the instrument portal, tearing of the flap during fixation, and loss of fixation. The authors checked the instrument portal location using a needle prior to introduction. The flap should be wide enough to be fixed without any tension. In order to counteract gravity and the pressure of the bowel in the standing position, the flap should be placed 2 cm dorsal to the vaginal ring. For stronger fixation, the staples should be placed perpendicularly to the tissue by the creation of an extra portal for the stapling device.
Patient Outcome
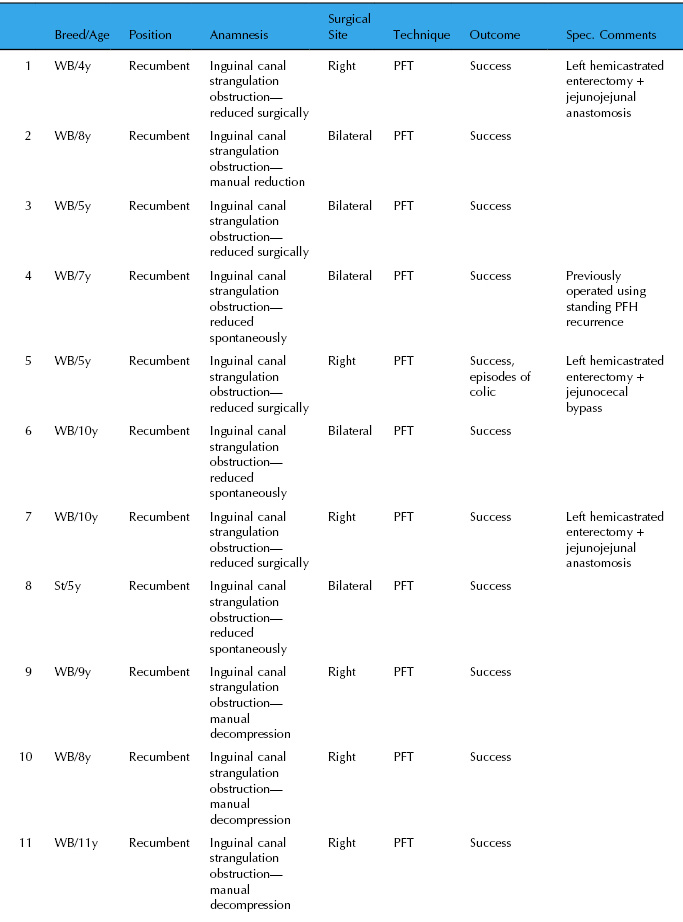
Fourteen horses were operated at the authors’ practices using the PFH technique under general anesthesia from 2003 to 2009. Details are summarized in Table 27.2. There was no recurrence of inguinal hernia in the 14 clinical cases (follow-up, 1–9 years). Five stallions were used for breeding with normal fertility; three were returned to high level show jumping; and one Standardbred performed as a racehorse. Five horses underwent rectal palpation and it was difficult or impossible to insert a finger into the inguinal canal. No morphological changes were noted in the scrotal area.
Table 27.2 Results of PFH in 14 horses operated 2003–2009
Sparsity of Available Literature
There are few papers describing the risk factors of inguinal herniation, the recurrence rate, or the outcome after inguinal hernioplasty or herniorrhaphy, and there are perhaps as many techniques as publications. Moreover, inguinal herniorrhaphy is not a very common condition and the statistical analysis of the outcome is difficult.
Closing the vaginal ring without castration is recommended for breeding stallions that have had inguinal herniation, whether or not it has been reduced surgically (Mariën 2001). The size of the vaginal ring may be correlated with the probability of inguinal herniation. In the six stallions described by Mariën (2001), all had enlarged and flaccid rings, as they did in our cases. We noted variation in the size and shape of the vaginal rings during standing laparoscopy. Larger, flaccid, funnel-shaped rings were observed, and it is our impression that these horses may have an increased risk of inguinal herniation.
Congenital inguinal herniation has been observed in foals. Typically, spontaneous, gradual reduction occurs as the foal grows so that by 12 months of age, the hernia disappears. This condition is genetically linked, and many breed organizations forbid breeding from affected horses (Cox 1987). Nonetheless, in severe cases, it is sufficient to simply close the vaginal ring by celioscopic stapling after reduction of the herniated viscera (Fischer 2002). In adult horses, because of the greater intra-abdominal forces that may occur, this approach may be insufficient (Fischer et al. 1995; Fischer 2002) and techniques that improve resistance to muscular forces associated with movement and intra-abdominal pressure may be necessary (Fischer 2002). Fischer et al. (1995) reported an adaptation of a human surgical technique known as transabdominal preperitoneal (TAPP) mesh repair for herniorrhaphy in two stallions in Trendelenburg position, under general anesthesia. Polypropylene mesh was positioned under a peritoneal flap, collapsing the neck of the vaginal tunic and decreasing the size of the vaginal ring. It was placed and secured in position with laparoscopic staples. Tissue ingrowth into the mesh led to the development of scar tissue and prevented reherniation. In our experience, mesh placement is time-consuming and the mesh has to be positioned very close to the spermatic cord to be effective. The TAPP technique is reportedly successful in the prevention of direct hernias in man by way of its resistance to high intra-abdominal pressure. It may be less well suited to the anatomy of the inguinal area in horses where indirect hernias are more frequent and where reinforcement of the peri-inguinal area seems unnecessary.
Adhesions after hernioplasty are described in man, although TAPP and total extraperitoneal (TEP) hernioplasty techniques decrease this risk (2.79% for TAPP, 0.57% for TEP techniques) (Bringman & Blomqvist 2004). In our clinical cases, the first horse underwent the TAPP technique. It was technically difficult to totally cover the mesh with peritoneum, and the horse developed adhesions postoperatively. Further dissection of the peritoneum laterally might have improved this coverage. Although PFH was successful in this horse, the dissection of the flap was more difficult during the second surgical intervention.
A simpler technique that involves insertion of a polypropylene mesh (rolled into a cylinder and maintained with two sutures) into the vaginal canal during standing laparoscopy has been reported by Mariën (2001). After insertion of the mesh into the inguinal canal, the proximal or both the proximal and distal sutures are cut using laparoscopic scissors, allowing the mesh to unfurl and fill the canal. The proximal part of the mesh is then secured to the parietal peritoneum using endoscopic staples. Subsequent granulation tissue is expected to obliterate the inguinal canal.
In both mesh techniques, the polypropylene is in close contact with the spermatic cord. In man, testicular atrophy (Wantz 1991) and infertility (Yavetz et al. 1991) have been reported after mesh implantation. This may be caused by ischemic orchitis, immunological reactions, or surgical trauma to the spermatic cord. Although good local tolerance has been reported in horses, sterility may likewise occur because of mesh contact with the spermatic cord, whereas there is no mesh implant with PFH. Thus, foreign body reaction or irritation of the spermatic cord should not occur with PFH. In a preliminary study, nine experimental horses were rechecked by standing laparoscopy 7 days after the first surgery. In these animals, the vaginal ring had been effectively and completely covered in all except Horse 1, where there was only partial coverage, probably because of lack of experience in the technique. In the others, the vaginal ring was no longer visible and it was impossible to enter the inguinal canal with an instrument. The peritoneal defect caused by the dissection of the flap was covered with scar tissue (Figure 27.5). The flap was generally weakly adhered to the spermatic cord, and this adhesion was easy to break by applying traction to the cord. Beneath the flap adhesion, the spermatic cord appeared normal. No adhesion was observed between the viscera, the flap, or its donor bed. There was the risk that surgical compression and irritation might occur after scar formation and wound contraction, but this was not observed clinically. Closing the vaginal ring in standing normal horses by apposition of the cranial and caudal edges, either by endoscopic staples or sutures, has been reported (Perrin 1997; Nudelmann 1999), but long-term outcome has not been described.
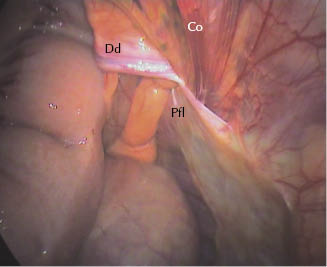
Figure 27.5 Left vaginal ring: view of the flap covering the vaginal ring 3 weeks postoperatively. Dd, deferent duct; Pfl, flap; Co, testicular cord.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree