CHAPTER 48 Infertility Due to Noninflammatory Abnormalities of the Tubular Reproductive Tract
Infertility in the cow can be attributed to a number of conditions affecting the tubular reproductive tract. Studies have confirmed that inflammatory disease (e.g., endometritis) is considerably more likely to compromise fertility than is noninflammatory disease.1, 2 In these limited surveys, noninflammatory lesions of the tubular tract accounted for approximately 5% of all lesions detected and were more prevalent among nulliparous animals. As expected, a larger proportion of nulliparous than of multiparous animals will be affected by noninflammatory lesions, in keeping with the absolute state of infertility in many congenital conditions. Although noninflammatory abnormalities are of relatively minimal importance overall, the cases provide for interesting discussion, particularly with regard to mode of development. The embryology of the reproductive system often is a critical factor in the pathogenesis of most such conditions, and the reader is encouraged to review the development of the female tract. This chapter focuses on several conditions that are responsible for infertility due to noninflammatory abnormalities of the tubular reproductive tract.
FREEMARTINISM
Freemartinism is the most commonly recognized noninflammatory condition resulting in infertility involving the tubular reproductive tract in the bovine. Freemartin heifers result from 92% of heterosexual twin births,3 and it is estimated that at least 86,000 freemartins are born annually in dairy cattle breeds in the United States.4
The development of the freemartin heifer is a result of the fusion of the chorioallantoic portions of the twin placentas, which usually is established between days 28 and 30 of gestation and results in a common blood supply between twin fetuses. This common supply allows the exchange of humoral and cellular elements between fetuses, producing calves that are blood cell chimeras. Chimeras are individual animals that contain two cell types originating from separate zygotes. Testicular development occurs before ovarian development in the bovine, and antimüllerian hormone (müllerianinhibiting substance) from the testes of the male fetus inhibits the development of the paramesonephric (müllerian) ducts in the female.5 This results in dysfunction in the differentiation of the ovaries, oviducts, uterus, cervix, and cranial vagina. The pathomechanism in 8% of the cases in which a fertile female is born as a twin to a male probably is failure of fusion of fetal membranes or timing of membrane fusion after a critical point in reproductive organ differentiation.6 The male co-twin usually also is chimeric and may exhibit deficient reproductive capacity.7 Approximately 6% of twin pregnancies result in the birth of a single calf,8 and in rare cases a female freemartin calf is born as an apparent singleton as a result of death of the male co-twin in utero.9 Freemartinism occasionally occurs in conjunction with other congenital abnormalities such as atresia recti10 and urethral hypoplasia.11, 12
Clinically, freemartin heifers exhibit broad variation in appearance of the external genitalia, with features ranging from apparent normality (most common) to a small vulva, increased anogenital distance, an enlarged clitoris, and a prominent tuft of hair on the ventral commissure of the vulva. The internal reproductive organs usually are abnormal, with vestigial or masculinization of the ovaries, reduced development of the paramesonephric (müllerian) duct system, and development of the mesonephric (wolffian) duct system. Freemartin females show a range in the internal reproductive organs dependent on the level of masculinization. The least masculinized form is most common, with a small genital tract with hypoplastic ovaries, a short vagina, and an absent cervix. Masculinization appears to be due to in part to a probably indirect role of antimüllerian hormone on gonadal development13 and the influences of H-Y antigen14 and results in ovaries with parenchyma that contains tubules and interstitial tissue, resembling testes. In animals that have undergone masculinization, development of the mesonephric (wolffian) ducts also occurs, which may result in epididymides, vasa deferentia, and vesicular glands.15 It is suggested that the extent of transformation of the female freemartin is dependent on the stage in gestation at which extensive anastomosis exposed her to circulating male hormones.16 Of interest, the proportion of male blood cells in circulation apparently has no relationship with the degree of masculinization in freemartin heifers.17
The diagnosis of freemartin heifers usually is based on a history of heterosexual multiple births, appearance of the external genitalia, and palpation or ultrasound evaluation of the internal reproductive tract. Visualization via vaginal speculum usually will reveal a short vagina and no external cervical os in freemartin heifers. The vagina in suspected freemartin heifers may be probed using a suitable instrument (a test tube, insemination pipette, or thermometer is commonly used); alternatively, commercial probes are available (Ru-an Freemartin Probe, $US25.95, Valleyvet.com). Freemartin heifers generally have a vagina that is approximately one-third the length of vaginas in normal heifers of the same age. Among heifers younger than 1 month of age, the freemartins usually will have a vagina length of 5 to 6 cm, whereas in normal heifers, vaginas are 13 to 15 cm long.18, 19 The vaginal length in suspected freemartin heifers should be compared with that in normal heifers of the same size. Occasional false positives using the vaginal length test can be due to interference by a persistent hymen with the deposition of the probe. False negatives also may be encountered, because some freemartins have a vagina that is of normal length. Eighty percent of freemartin heifers will be correctly identified using a vaginal probe,20, 21 with the remaining 20% of heifers likely to consist of approximately half freemartins and half fertile heifers. It is suggested that laboratory testing be carried out on the heterosexual twin heifers with normal vaginal length.
Laboratory testing includes polymerase chain reaction (PCR) analysis, karyotyping, blood typing, and the erythrocyte lysis test for the detection of blood cell chimeras. Although most female cattle that are blood cell chimeras are likely to be sterile freemartins, several fertile XX/XY animals have been described.22–25
PCR assay has become the most common commercially available test to diagnose freemartins. The PCR procedure demonstrates sex chromosomes exhibiting XY and XX in the same animal. The test is relatively fast and highly accurate because it can detect the presence of as little as 1 in 10,000 blood cells containing the Y chromosome.26 A blood tube containing 5 to 8 ml of whole blood with ethylenediaminetetra-acetic acid (EDTA) or a drop of blood on a blood card from a suspected freemartin may be submitted for testing. The cost for one assay in 2005 was $US40. This test is available from several laboratories including Biogenetic Services Inc., Brookings, SD (info@biogeneticservices.com), and Veterinary Genetics Laboratory, Davis, CA (cattle@vgl.ucdavis.edu).
Karyotyping is another method for demonstrating blood cell chimerism. Blood lymphocytes are cultured and metaphase chromosome spreads are examined for XY cells. The distribution of male cell percentages in nucleated blood cells appears to be random in individual animals, and freemartins may contain a high to low percentage of XY cells.27 False negative results can occur in animals containing a small proportion of XY cells if insufficient metaphases are counted. Karyotyping requires considerable laboratory work and is fairly expensive. Another interesting test of XX/XY chimerism is through skin grafting between heterosexual twins—freemartin heifers accept a surgically placed skin graft from their male twin.28
OTHER INTERSEX CONDITIONS RESEMBLING FREEMARTINISM
Intersex cattle other than freemartins are very rare. Male pseudohermaphrodites are genetic and gonadal males, but the external genitalia resemble those of a female. Male pseudohermaphrodites are more common than female or true hermaphrodites, probably because more genes are required to initiate male development (steroidogenic enzymes, 5α;–reductase, and androgen receptors), with correspondingly greater opportunity for genetic defects. Limited case reports of male pseudohermaphrodites in cattle have been published.29–32 Classification of intersex cases requires careful anatomic (often at post mortem), chromosomal, and endocrinologic examination. It is possible that some other intersex conditions are misclassified as freemartins if the diagnosis is based solely on physical examination.33
Bovine male pseudohermaphroditism (often referred to as testicular feminization) results from androgen insensitivity. Affected animals are XY males with testes, paramesonephric and mesonephric (wolffian) duct regression, and female-like external genitalia. The tubular genitalia of the paramesonephric (müllerian) system are underdeveloped owing to testicular production of antimüllerian hormone (müllerianinhibiting substance). Production of testosterone by the testes is normal, but because of intracellular androgen insensitivity, the mesonephric (wolffian) system fails to develop without androgenic support. It is possible that this disorder is inherited as an X-linked trait in cattle, as in other species.31 Clinically, affected animals may be mistaken for heifers but fail to show estrus and exhibit bull-like behavior. On examination of the internal reproductive tract, the vagina may be short to normal in length; no cervix is present, with a very small or absent uterus and testes in the normal position of ovaries.32
SEGMENTAL APLASIA OF THE PARAMESONEPHRIC (MÜLLERIAN) DUCTS
Segmental aplasia is a result of a prenatal lack of development of a portion of the paramesonephric (müllerian) duct system, resulting in various degrees of aplasia, potentially involving the vagina, cervix, uterus, and oviducts. Historically described as “white heifer disease”34, 35 owing to a high prevalence in white Shorthorn females, segmental aplasia has been reported in several other breeds of cattle including Holsteins,1, 36 Senepol,37 German Black Pied,38 Guernsey,1 Jersey,1 Angus,39 and Ayrshire.39 The prevalence of segmental aplasia ranges from 0.15%1 to 0.2%40 in slaughterhouse studies. Although rarely diagnosed in practice, segmental aplasia in cattle provides an often academic discussion of the locally acting nature of endometrial prostaglandins, the likely inheritance of the condition, and cull versus treatment options.
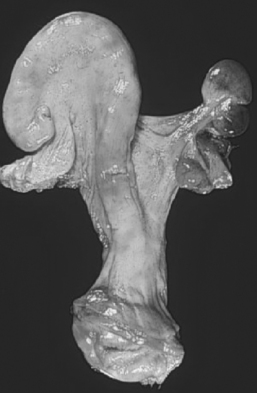
A range of defects of the paramesonephric (müllerian) system may be seen in animals affected by segmental aplasia. Individual cattle may present with abnormalities ranging from an imperforate hymen to complete maldevelopment of the tubular reproductive tract. The most common presentation encountered in practice is aplasia and resultant blockage affecting the caudal portion of one uterine horn, often referred to as uterus unicornis, resulting in accumulation of endometrial secretions in the cranial horn (Fig. 48-1). In a review of 20 cases of uterus unicornis, 14 animals had a defective right uterine horn and 6 had a defective left horn.39 A persistent hymen usually is not present.39 When both horns are affected, the aplasia includes the cervix and vagina.41 Segmental aplasia of the oviducts is rare,2 and obstruction of the oviducts may result in accumulation of secretions and hydrosalpinx. The aplastic section of the reproductive tract usually consists of a band of connective tissue and muscle with no lumen, mucosal epithelium, or glands.36, 41
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree