Target antigen
Host species (label)
Clone
Dilution
Comment
Mouse CD3
Hamster
500A2
1:100
Permeabilization step required
Mouse CD4
Rat
H129.19
1:100
Mouse CD8
Rat
53-6.7
1:100
Mouse FoxP3
Rat
FJK-16s
1:50
Permeabilization step required
Mouse I-Ab
Mouse (biotinylated)
KH74
1:300
Mouse CD19
Rat
1D3
1:100
Table 2
Suggested secondary antibodies
Target antibody | Host species (label) | Cat. no. | Source | Dilution |
|---|---|---|---|---|
Rat IgG | Rabbit (biotinylated) | BA-4001 | Vector | 1:200 |
Hamster IgG | Goat (biotinylated) | BA-9100 | Vector | 1:200 |
Table 3
Suggested streptavidin conjugates
Target | Conjugates | Cat. no. | Source | Dilution |
|---|---|---|---|---|
Biotin | Streptavidin-DyLight 488 | SA-5488 | Vector | 1:400 |
Biotin | Streptavidin-DyLight 594 | SA-5594 | Vector | 1:400 |
19.
Phosphate-buffered saline (PBS): 1.54 mM KH2PO4, 155.17 mM NaCl and 2.71 mM Na2HPO4, pH 7.4, sterilized by autoclaving.
20.
Tepid water.
3 Methods
3.1 Sectioning of Aortic Roots and Slide Preparation
In order to optimize the results of the immunostaining, the collection, preservation, and processing of organs should be performed in a standardized way.
1.
After dissection, the hearts should be kept in PBS on ice until the mounting, or be directly mounted in mounting medium and being snap frozen. The embedded hearts should be kept at −80 °C until the cryosectioning is performed.
2.
For sectioning of the aortic roots, it is recommended to use the method described by Nicoletti et al. [7]. Briefly, the proximal 800 μm of the aortic root is serially sectioned on a cryostat, with each section 10 μm thick. “Point Zero,” where the aortic root begins, is located where the cusps of the aortic valves start to be visible and is found by sectioning the heart in an upstanding position in a caudal-to-cranial direction. From this point, 10 μm thick sections, starting at the 90–880 μm levels (distance from “Point Zero”) are collected to slides following scheme of Fig. 1. The first 90 μm of sections are used to adjust the angle to get all three cusps visible and aligned.
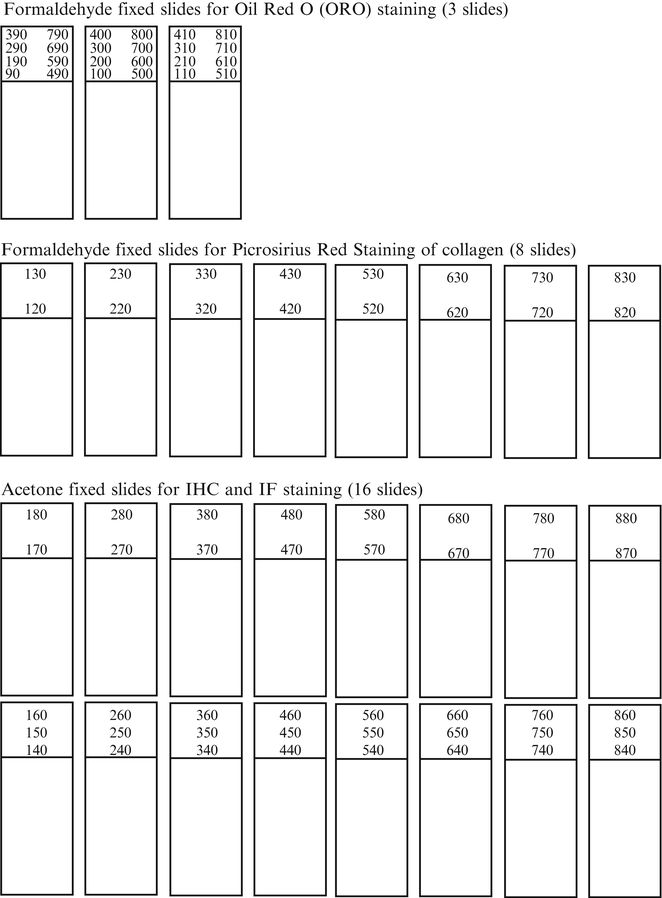

Fig. 1
Template for aortic root sectioning and organization of slides
3.2 Tissue Fixation
2.
Remove the slides from fixation reagent and let them dry, at room temperature, for 1 h.
3.
After fixation, slides should be stored at −20 °C until staining.
3.3 Immunostaining
3.3.1 Immunohistochemistry of Acetone-Fixed Frozen Sections
All steps are conducted in room temperature unless otherwise stated.
5.
Incubate sections with avidin solution (from Avidin and Biotin blocking solution, Vector, item 8 in Subheading 2) for 15 min. Rinse briefly after incubation.
6.
Incubate sections with biotin solution (from Avidin and Biotin blocking solution, Vector, item 8 in Subheading 2) for 15 min.
7.
Rinse with rinsing buffer, three times for 5 min.
8.
Block the sections with staining buffer for 30 min. Remove the excess of staining buffer from sections, but do not rinse the slides after the incubation.
10.
Incubate the slides overnight at 4 °C, or 1 h at room temperature.
11.
Rinse with rinsing buffer, three times for 5 min.
12.




Add secondary antibody (biotinylated) diluted in staining buffer. Incubate the slides for 30 min.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


