CHAPTER 14 Immunologic Development and Immunization
Overview of Immunity of the Puppy and Kitten
Puppies
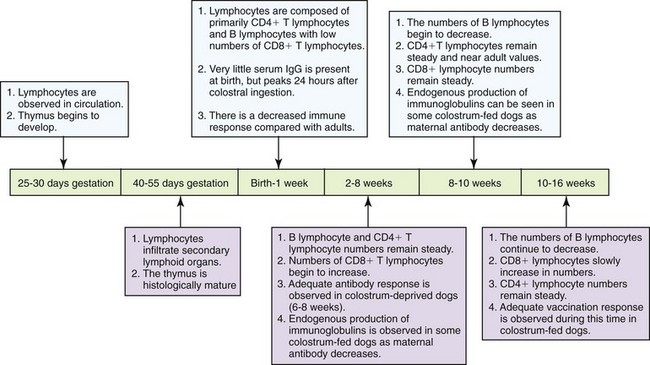
Immunologic development of the lymphoid system is incremental in all species. As a generality, the shorter the gestation, the less developed the immune system is at birth. In puppies, immunologic development is an early event, beginning in midgestation with lymphocytes present in circulation at 25 days of gestation (Figure 14-1). Thymic development begins on day 27 of gestation, followed by lymphoid infiltration into secondary lymphoid organs, including spleen and lymph nodes, on days 45 to 52 of gestation. Thymic development appears histologically normal by day 45 of gestation. In contrast, splenic and lymph node architecture is not completely developed in the fetus and lacks germinal centers and B cell follicles until shortly after birth.
Concepts of Maternal Immunity and Temporary Pregnancy-Associated Immune Suppression
The outcomes of the temporary immunodeficiency states allow for fetal survival but may also result in an increased susceptibility to environmental infections by bacteria and fungi. Intracellular infections, such as viruses and protozoans, which may have been acquired during postnatal development, may become exacerbated during pregnancy because of the suppressive effects on the Th1 cells. This suppression results in a decreased CMI response. The CMI effector cells, the T cytotoxic lymphocytes, function normally to control virtually all the viral infections such as canine herpesvirus. Intracellular bacterial infections, such as Brucella canis, become more pathogenic during pregnancy. In addition to the aforementioned effects on T cell function, macrophage function is also compromised, allowing opportunistic bacteria, which are usually confined to external mucosal surfaces of the body, to become systemic. Concurrent with the immunosuppression that accompanies pregnancy, there is an increased shedding of infectious microorganisms. This is considered to be an extension of impaired T cytotoxic lymphocyte function. Although the pregnant animal may appear clinically normal, her altered immune response results in an increased shedding of gastrointestinal viruses, such as canine coronavirus and canine rotavirus, and bacteria, such as Escherichia coli, usually during the periparturient period. This increase in shedding of infectious microorganisms is an important factor when addressing the issues of management of animals through this period of time (Figure 14-2). The suppression of neutrophil functions during later stages of pregnancy and immediately postpartum are the subject of active investigation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree