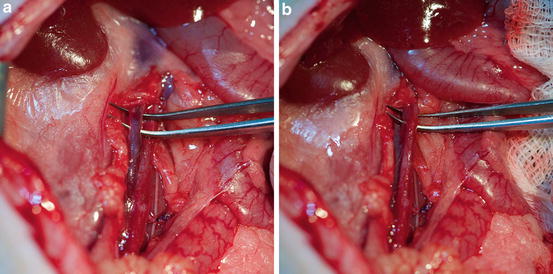
Fig. 9.1
Heterotopic heart transplantation – comlete volume unloading
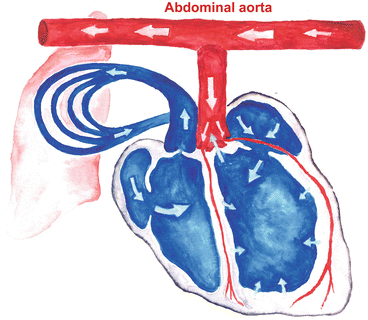
Fig. 9.2
Heterotopic heart transplantation – partial volume unloading
9.2 Preconditioning of the Animal Prior to Surgery
It is recommended to allow the animals to become familiar with the facility equipment and staff for at least 2 weeks after arrival from the supplier. This can provide the animals the necessary time to get accustomed to the environment and reduce stress. An added benefit is that the researcher can become familiar with individual animals, can identify those that are not in a perfect health condition and prevent them from inclusion into the experimental group. Ultimately, the animals used in chronic studies must be housed for at least 72 h prior to any procedures being performed.
Each animal must be clearly identifiable during the all phases of the study – they have to be explicitly marked before the first manipulation. Rats are unable to vomit, thus it is not absolutely required to let them fast before the surgery. But, in order to reduce the volume of intestinal content and therefore make the surgical manipulations within abdominal cavity easier, overnight fasting is recommended. This fasting does not mean starvation. Thus, in order to prevent ketosis and hypoglycemia, food pellets should be replaced by glucose solution and water freely accessible for the animal.
Both approaches for total anesthesia induction and maintenance can be utilized according to the routine of the researcher. Inhalation anesthesia used for heart donors is performed with a mixture (3–5 %) of isoflurane in the oxygen throughout the duration of the surgery. Recipient anesthesia can be based on thiopental (50 mg/kg) diluted in 1 ml warm saline and administered intraperitoneally.
During the anesthesia, temperature maintenance is crucial – the animal should be placed on safe heating pad, the room temperature should not be under 22 °C and the saline used for visceral moisturizing must be warmed to 37 °C.
9.2.1 DO’s Just Before the Surgery
1.
Check the donor visually, weight it and calculate the dose of anesthetic drugs.
2.
Inject the anesthesia and let the animal sleep in the dark.
3.
Donor anesthesia consists of isoflurane at 5 % for induction and 1.5 % for maintenance administered via the nose cone.
4.
Shave an area large enough to include a complete incision line and some surrounding skin allowing efficient disinfection.
5.
Treat the skin using detergent ⇒ alcohol ⇒ disinfectant.
6.
Cover donor eyes with a proper unguent and put it onto the heating pad with the backside down.
7.
Drape the animal using the sterile cloth to provide a sterile field around the incision
8.
Intraoperative pain relief consist of a single bolus dose of buprenorfine (10 μg/kg) given via the intramuscular route
9.3 Heterotopic Abdominal Heart Transplantation
Heart transplantation in this manner leads to complete mechanical unloading of a donor heart . The heterotopic abdominal heart transplantation consists of anastomosis of the donor aorta to the syngeneic recipient aorta and the donor pulmonary artery to the recipient inferior vena cava. Blood flows from the recipient aorta into the transplanted aortic root. Due to the aortic valve competency, blood perfuses the coronaries without entering the left ventricle. Coronary flow is returned into the right atrium via the coronary sinus and then ejected by the right ventricle through the pulmonary artery. Only thebesian circulation allows a minute amount of blood to enter the left ventricle (Fig. 9.1).
9.3.1 Recipient Preparation
9.3.1.1 Equipment + Material
Microsurgical Instruments
Scissors, forceps, 7-0 silk suture, two micro-scissors (straight, curved), three micro-forceps (two straight, one curved), thermal cauterize unit, cotton swabs, gauze, warm saline solution, heating pad, needles, syringes (5 ml, 10 ml).
9.3.1.2 Procedure
1.
Open abdominal cavity with a midline incision.
2.
Cauterize capillary bleeding from the incision edge and insert abdominal retractors.
3.
Carefully retract the bowels to the animal left side and wrap them in wet gauze (Fig. 9.3).
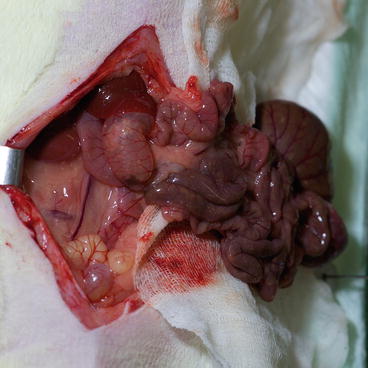

Fig. 9.3
Recipient – abdominal cavity opening
4.
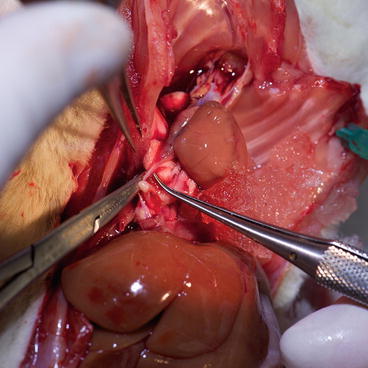
Dissect the retroperitoneal fascia using wet cotton swabs and expose the infrarenal portion of the great vessels for a length of 4 cm. Later, using two forceps (one straight, one curved), carefully dissect around great vessels and underneath them. It is necessary to ligate all (even minor) branches with 7-0 silk (Fig. 9.4).
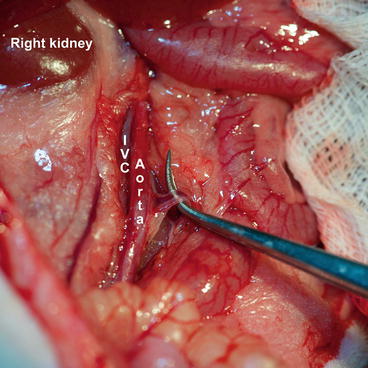

Fig. 9.4
Recipient – aortic side branches ligation
5.
6.
Return the bowels into the abdomen and wet them with warm saline during donor harvesting operation.
9.3.1.3 Tips and Tricks
1.
Wash the serosa with warm saline (37 °C) so that the tissue will not be sticky for surgical instruments.
2.
Prepare the recipient as described above to minimize the cold ischemia time of the graft.
3.
Carefully expose and ligate all branches in the desired area to prevent bleeding after vessel opening.
9.3.2 Heart Graft Procurement
9.3.2.1 Equipment + Material
Microsurgical Instruments
Scissors, forceps, fine mosquito hemostat, 7-0 and 5-0 silk suture, two micro-scissors (straight, curved), three micro-forceps (two straight, one curved), cotton swabs, gauze, cold saline solution and ice, needles, syringes (5 ml, 10 ml), heparin (5000 I.U.) diluted in 5 ml of cold saline solution, cold cardioplegic solution (St. Thomas).
9.3.2.2 Procedure
1.
Open abdominal cavity with a midline incision and expose the great vessels as before.
2.
Use a 5 ml syringe and, via a needle, inspire approximately 5 ml of blood from the abdominal aorta. Immediately close the puncture site with hemostat.
3.
Administer heparin (5000 I.U.) diluted in cold saline into the inferior vena cava via a 30-g needle (Fig. 9.6) and close the puncture site with hemostat as well.
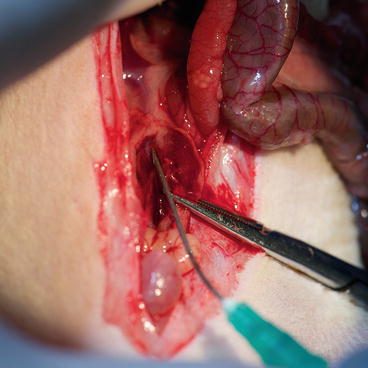

Fig. 9.6
Donor – heparin administration via the abdominal inferior vena cava
4.
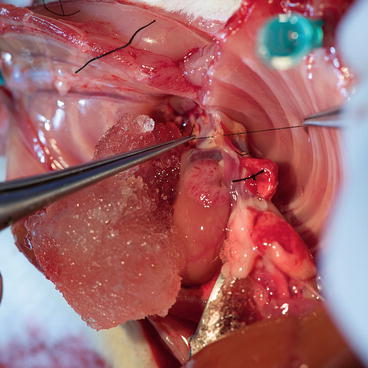
Perform the midline sternotomy; expose the aortic arch by dissecting the thymus. Using fine forceps, clean the innominate artery (Fig. 9.7) and place a long silk suture around, which can be used to ease the innominate artery catheterization with a blunt 30-g cannula and also seal it using tourniquet or ligation.
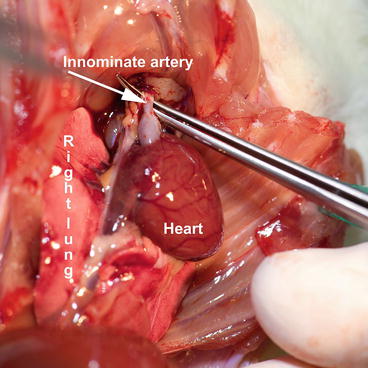

Fig. 9.7
Donor – situation after sternotomy
5.
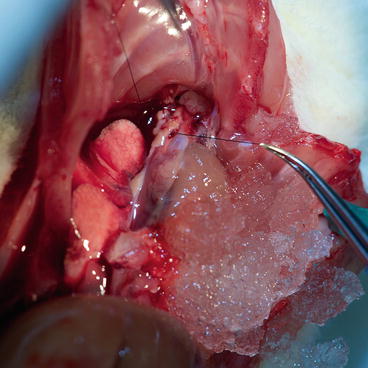
Using fine straight scissors, open the innominate artery, insert the blunt cannula heading to the aortic root and tighten the tourniquet (Fig. 9.8).
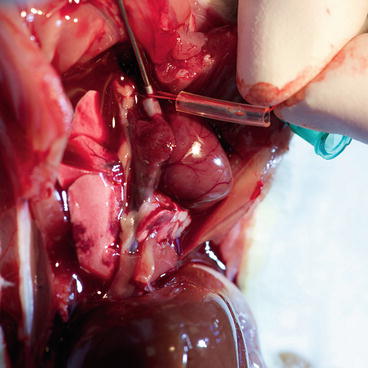

Fig. 9.8
Donor – cannula insertion into the ascending aorta
6.
Clamp the rest of aortic arch with hemostat and arrest the heart by administering 50 ml cold cardioplegic solution very slowly via the aortic cannula and control the coronary vessels washout with cardioplegic solution. Meanwhile, cut superior and inferior vena cava to prevent heart extensive filling and distention. As soon as possible, apply ice on the heart (Fig. 9.9). After heart arrest, take the cannula out from the brachiocephalic trunk.
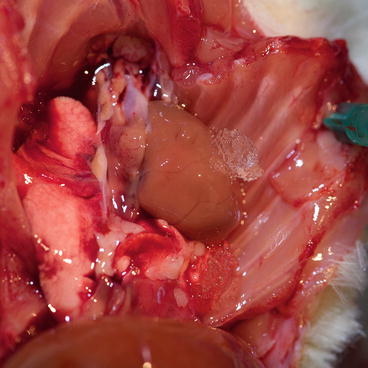

Fig. 9.9
Apply ice on the heart
7.
Ligate with a silk 5-0 suture right and left lung close to the hilus and cut both lungs away (Fig. 9.10).


Fig. 9.10
Ligate with a silk 5-0 suture right and left lung close to the hilus
8.
9.
Clean the aorta and pulmonary artery. The aorta should be transected, just before the brachiocephalic trunk (Fig. 9.14), and the pulmonary artery, just below its bifurcation (Fig. 9.15), in one cut (straight microsurgical scissors).
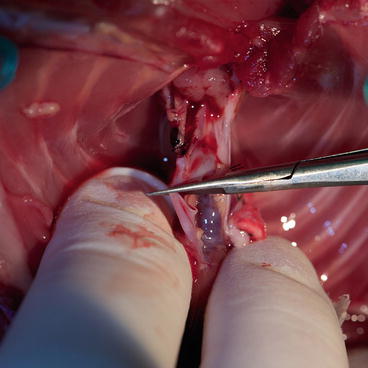
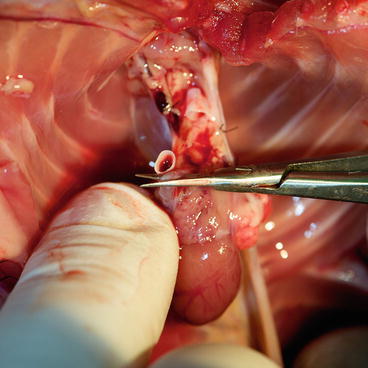

Fig. 9.14
The aorta should be transected, just before the brachiocephalic trunk

Fig. 9.15
Transect the pulmonary artery just below its bifurcation
10.
Keep the heart in ice-cold cardioplegic solution until reimplantation.
9.3.2.3 Tips and Tricks
1.




Be careful about any air in the needle or syringe during the administration of heparin or cardioplegia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree