CHAPTER 15 HAMSTERS AND GERBILS
COMMON SPECIES KEPT IN CAPTIVITY
Hamsters
In addition to the golden or Syrian hamster (Mesocricetus auratus), which is the most common hamster used in research and maintained as a pet, there are several other species that the clinician may encounter. These include the Siberian or Djungarian hamster (Phodopus sungorus), the Chinese or striped hamster (Cricetulus griseus), the common black-bellied or European hamster (C. cricetus), and the gray Armenian hamster (C. migratorius). Hamsters are members of the family Muridae, subfamily Cricetinae, and comprise five genera dispersed across Europe and Asia.1 In total there are 24 species of hamster in five genera found across Europe and Asia. Of these species in the wild, the golden hamster (M. auratus), of northwestern Syria, and Calomyscus hotsoni are endangered, and M. newtoni is considered vulnerable.1
The golden hamster is the largest of the common pet species, with an average body weight of 120 g. The golden hamster was originally described from a single specimen collected in 1839. In 1930, a female and her young were found in Syria, taken to Israel, and then bred and distributed to England and the United States.1 All golden hamsters in captivity today descended from these original 13 hamsters captured in Syria. The golden hamster is nocturnal, solitary, and known for its pugnacious tendencies. Today, captive golden hamsters are primarily found in the wild-type or reddish-golden brown hair coat with gray-white ventrum. Other coat color variations are the result of genetic mutation and include cinnamon, very dark brown, cream, white, piebald, albino, and long-haired “teddy bear” hair coats.2
Other hamster species commonly found in the pet trade are the Chinese hamster and the Siberian or dwarf hamster. Chinese hamsters were brought to the United States in 1948 and domesticated and reproduced in captivity beginning in 1949-1952.3 Chinese hamsters, also known as striped back hamsters, are dark brown and smaller than Syrian hamsters. These hamsters are aggressive, solitary, and nocturnal with a body weight of 30 to 35 g; males are larger than females. Chinese hamsters have the lowest number of chromosomes (11; 2N = 22) of any lab animal but are difficult to breed in captivity.3 Chinese hamsters are used as lab animal models for cytogenetics, diabetes mellitus, and toxicology research investigations.
Siberian hamsters are dwarf hamsters with an average body weight of 40 g. These hamsters have a gray pelage dorsally with a dorsal midline black stripe and a white ventrum. Also known as the Dzungarian hamster, this species is relatively tame and nonaggresive compared with other hamsters. Unlike Syrian hamsters, this species is colonial and can be housed in family groups.4 Male hamsters will attend to the young. Dzungarian hamsters are used for research of photoperiod, the pineal gland, and thermoregulation.
Gerbils
The Mongolian gerbil (Meriones unguiculatus), more correctly called the Mongolian jird, is also known as the clawed jird, the sand rat, or the desert rat.5,6 This gerbil is one of 95 species and 14 genera in the order Rodentia, family Muridae, subfam ily Gerbillinae, which are all correctly called gerbils.6 Gerbils are the world’s largest group of rodents adapted to arid environments, which range from Africa though the Middle East to Asia.6 Of the 95 extant species of gerbils, 18 are endangered and 4 species are vulnerable to extinction.
M. unguiculatus, the common captive species of gerbil, is a burrowing diurnal rodent with crepuscular tendencies (Figure 15-1). This is a hot-desert-dwelling, social species native to Mongolia and northeast China. Agouti is the “wild type” and most common pelage color, but gerbil pelage color may also be black, gray, piebald, blue, Burmese, Himalayan, silver, or white.4 Agouti animals have darkly pigmented skin with a light buff to white ventrum and mixed white, yellow, and black hairs dorsally, giving the animal a mixed brown appearance.5 Adult M. unguiculatus weigh 70 to 150 g, and males are 5% to 10% heavier than females.4,5 The Libyan gerbil, M. libycus, is also maintained as a pet and has similar attributes. Most gerbils live about 2 to 3 years, but a 5-year life span may occur; females tend to live longer than males.5,7
BIOLOGY
Unique Anatomy and Physiology
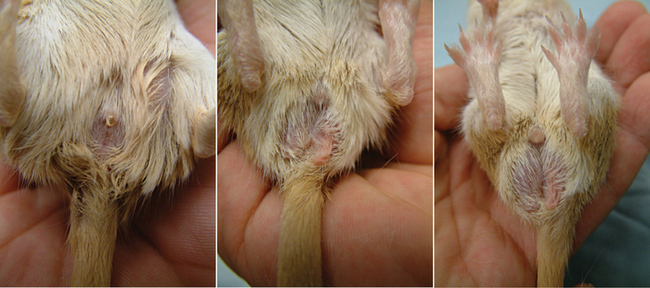
Hamsters and gerbils are rodents and have common dentition: I 1/1, C 0/0, PM 0/0, M 3/3.8 The crown-to-length ratio of upper to lower incisors is 1 : 3.8 The lack of canines and premolars creates a gap between the molars and incisors called the diastema. The incisors are open rooted and grow continuously, whereas the molars are not open rooted. Rodents cannot sweat or pant; they dissipate excess heat primarily through their tail and ears.8 Thus, they are susceptible to heat stress and require controlled environmental temperatures. Both hamsters and gerbils are polyestrous. Gender determination is based on an increased anogenital distance in the male (Figure 15-2). The penis of rodents, including the hamster and gerbil, has a baculum (os penis). In general, the anatomic and physiologic parameters of gerbils and the golden hamster are similar (Table 15-1).
TABLE 15-1 Biodata of Hamsters and Gerbils
| Parameter | Hamster | Gerbil |
|---|---|---|
| Body temperature (° C) | 37-38 | 38-39 |
| Respiratory rate (breaths/min) | 38-110 | 85-160 |
| Blood volume (ml/100 g body weight) | 6.5-8.0 | 7.7 |
| Heart rate (beats/min) | 310-471 | 260-600 |
| Adult body weight (g) | ||
| Male | 87-130 | 46-131 |
| Female | 95-130 | 50-55 |
| Life span (mo) | ||
| Average | 18-24 | 24-39 |
| Maximum | 36 | 60 |
| Food consumption/100 g body weight/day (g) | 8-12 | |
| Water consumption/100 g body weight/day (ml) | 8-10 | |
| Preferred environmental temperature range (° C) | 21-24 | 18-22 |
| Preferred environmental relative humidity (%) | 40-60 | 45-55 |
Data from Battles A: The biology, care and diseases of the Syrian hamster, Vet Compend Cont Ed 7(10):815-825, 1985; Harkness J, Wagner J: The Biology and Medicine of Rabbits and Rodents, ed 4, Baltimore, 1995, Lippincott Williams and Wilkins; Bihun C, Bauck L: Basic anatomy, physiology, husbandry and clinical techniques. In Quesenberry KE, Carpenter JW, editors: Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery, ed 2, St Louis, 2003, WB Saunders.
Hamsters
Multiple texts, including an anatomic atlas, are available for detailed study of the anatomy of the hamster.9 The following anatomic description should be considered only a summary of clinical anatomy of the Syrian hamster. The body of the Syrian hamster is short and stocky, about 15 to 20 cm long.2 Adult weights range from 110 to 140 g, with females being larger, on average, than males.2 Hamsters have short legs, thick fur, large ears, prominent dark eyes, long whiskers, and sharp claws. The paws of the front limbs of hamsters are modified hands with five digits. This adaptation affords dexterity for food and bedding manipulation.1 The rear foot has three toes.3
Hamsters have sebaceous “hip” glands on either flank which are used to mark territory and stimulate mating behaviors.4 These dark brown patches are covered by the fur and are larger in males than in females.4 In the sexually aroused male, these glands become readily visible as secretions cause the overlying fur to become matted.3
Hamsters have a movable mandibular symphysis which may not close during the hamster’s life span.4 The molars may retain food particles and are prone to dental caries.4 Newborn hamsters have fully erupted incisors for use in nursing.4 Cheek or buccal pouches of the hamster are used for storage and transport of food and bedding. These pouches evaginate from the lateral cheek at the diastema and extend alongside the head and neck to the scapulae.1,4 These thin walled, highly distensible, muscular sacs measure 35 to 40 mm × 4 to 8 mm × 20 mm and are lined with stratified squamous epithelium and are well vascularized.4 To empty these pouches, hamsters squeeze the cheek pouch contents forward with the paws.1 Regurgitation or vomition is unlikely in these species because of a limiting ridge at the junction of the esophagus and stomach. The hamster stomach has squamous and glandular portions and some fermentation occurs in the squamous forestomach.4
Gender determination, as in other rodents, is based on an increased anogenital distance in the male. In addition, the adult female has a more blunt posterior and 6 pairs of nipples, whereas the male has a more pronounced posterior bulge because of the testes in the scrotum.1,2 Reproductive characteristics of the hamster are summarized in Table 15-2.
TABLE 15-2 Reproductive Data for the Hamster and Gerbil
| Parameter | Hamster | Gerbil |
|---|---|---|
| Breeding onset | ||
| Male | 70-98 days | 70-85 days |
| Female | 42-70 days | 65-85 days |
| Cycle length | 4 days | 4-6 days |
| Gestation | 15-16 days | 24-46 days |
| Lactating 27-48 days | ||
| Postpartum estrus | No | Yes |
| Litter size | 5-9 | 3-7 |
| Birth weight | 2 g | 2-3 g |
| Weaning age | 20-25 days | 20-26 days |
| Breeding duration | 10-12 mo | 12-17 mo |
Data from Battles A: The biology, care and diseases of the Syrian hamster, Vet Compend Cont Ed 7(10):815-825, 1985; Harkness J, Wagner J: The Biology and Medicine of Rabbits and Rodents, ed 4, Baltimore, 1995, Lippincott Williams and Wilkins; Motzel S, Wagner J: Mongolian gerbils: care, diseases and use in research. In Dahm L, J MB, editors: Laboratory Animal Medicine and Science, Series II, Seattle, 2001, University of Washington; Bihun C, Bauck L: Basic anatomy, physiology, husbandry and clinical techniques. In Quesenberry KE, Carpenter JW, editors: Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery, ed 2, St. Louis, 2003, WB Saunders.
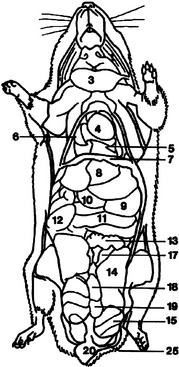
The brain has a smooth cerebrum with generalized areas of function and large olfactory bulbs. Hamsters have acute hearing and olfaction, thus communication is achieved through sounds audible to the human ear, through ultrasound, and through scent.1 Hamsters recognize other like individuals through scent and receptive mates by odor. Figures 15-3 and 15-4 overview the important anatomic features of the hamster.
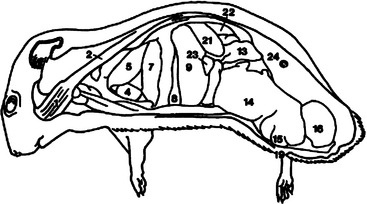
Figure 15-3 Lateral anatomic diagram of the internal anatomy of the adult male Chinese hamster (Cricetus griseus).
(Reprinted with permission from Gabrisch K, Grimm F, Isenbugel E et al, editors: Diagnostic Radiology of Exotic Pets, Philadelphia, 1991, WB Saunders.)
Gerbils
Gerbils are characterized by their long fully furred tail, strong claws for burrowing, and elongate muscular hindlimbs for leaping and standing upright.4,5 The body and tail each range from about 11 to 15 cm in length, with the body being a bit longer than the tail.5 Gerbils have multiple anatomic adaptations to help them avoid predation. The large middle ear enables them to hear low-frequency sounds, such as the owl wing beat.6 Gerbils have a wide field of vision because of the dorsal placement of their large eyes.6 The tail tip has a contrasting color of fur, which may distract predators; furthermore, the tail may be detached from the animal or degloved by the predator while allowing the gerbil to escape. This characteristic tail slip is of particular importance to the veterinarian; avoid holding gerbils by the distal tail to avoid tail degloving or amputation in the exam room.
The gerbil does not sweat and produces extremely concentrated urine as an adaptation for living in arid environments.6 The relatively large adrenal glands may also be adaptive for arid environments.4 All gerbils have a tan hairless elliptical area in the midventral abdomen that is a scent marking gland. This prominent abdominal pad is composed of large sebaceous glands that are responsive to testosterone and are therefore larger in the male.4 The female gerbil has two pairs of thoracic teats and two pairs of inguinal teats. Gerbils have a thoracic thymus, which persists into adulthood.4 The internal anatomy of the gerbil is detailed in Figures 15-5 and 15-6.
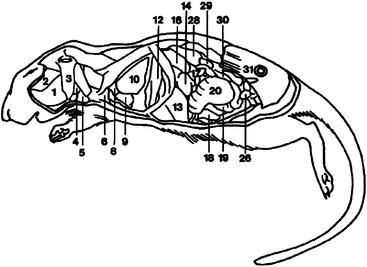
Figure 15-5 Lateral diagram of internal anatomy of the female gerbil (Meriones unguiculatus).
(Reprinted with permission from Gabrisch K, Grimm F, Isenbugel E et al, editors: Diagnostic Radiology of Exotic Pets, Philadelphia, 1991, WB Saunders.)
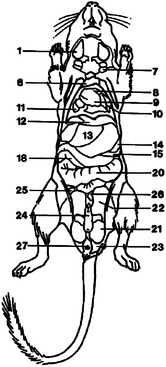
Figure 15-6 Ventral diagram of the anatomy of the male Mongolian gerbil (Meriones unguiculatus).
(Reprinted with permission from Gabrisch K, Grimm F, Isenbugel E et al, editors: Diagnostic Radiology of Exotic Pets, Philadelphia, 1991, WB Saunders.)
Like other rodents, the gender is determined in gerbils by anogenital distance, which is greater in the male. The increased prominence of the scent marking gland and the darkly pigmented scrotum is also helpful in sexing adult males.5 The neonatal gerbil may be gender determined based on the increased anogenital distance and the more prominent genital papilla of the male.5 Gerbils have 7 cervical, 13 thoracic, 6 lumbar, 4 sacral, and 7 or more coccygeal vertebrae (C7, T13, L6, S4, Cy7+). The gerbil has a nonglandular forestomach, lined with keratinized squamous epithelium, and a glandular stomach.
Gerbils form lifelong monogamous pair bonds. Male gerbils need not be removed from the enclosure when the litter is born, as aggression may ensue upon his reintroduction. Gerbils are polyestrous spontaneous ovulators with an estrous cycle of 4 to 6 days. Gestation in the gerbil can be prolonged by delayed implantation of postpartum estrus.5 For a summary of reproductive characteristics of the gerbil, refer to Table 15-2.
Wild gerbil reproductive behavior differs from that of other rodents. These animals live in large social groups of up to three adult males, up to seven adult females, and numerous subadults and juveniles in a single burrow system. Territories are strictly maintained, food is communally foraged and stored, and strange gerbils are chased away. However, the stable territorial group of gerbils that breed only among themselves would be subject to a variety of genetic problems associated with inbreeding. To avoid this issue, the female gerbil in estrus leaves her territory and mates with a male from another gerbil community.6 The female then returns to her own burrow where she raises her offspring along with her relatives.
HUSBANDRY
Enclosures for hamsters and gerbils should be easy to clean, be lightweight, and have a plastic bottom and sides deep enough to contain bedding. Additionally, these enclosures should be well ventilated. A large door or doors should be provided for removal of the animal from the cage. Small plastic heavy dishes are recommended for food and sipper bottles for water. Water deprivation is a common problem in pet rodents and should be avoided by inspection of sipper bottles or water containers daily for function, water level, and cleanliness. Although sipper water bottles are preferred, they can leak and become blocked with air bubbles, food material, or bedding material.
Exercise wheels and spherical exercise balls can be provided for exercise. Hamsters are well known for using exercise wheels, with female hamsters running up to 10 km/night.8 Long-haired hamsters should have their hair trimmed to avoid entanglement in their exercise wheels. Spherical exercise balls are also favored by the hamster, but owners should protect the hamster from stairs, direct sunshine, small children, and other pets.
Hamsters
Hamsters are relatively asocial and are best maintained as a solitary animal. Thus, housing groups of hamsters or introducing a new hamster into another hamster’s cage is not recommended, as cagemate trauma will likely result. Hamsters are escape artists and can chew through wood, soft metal, and plastic. The enclosure therefore must be well constructed, have rounded corners and be securely fastened. A dark place to hide (e.g., a can) is recommended.3 Adequate space for an adult hamster’s enclosure is 20″ × 20″ × 6-10″ in height.3 A 12-hour light cycle is preferred, and a light cycle of 14 hours light and 10 hours dark is optimal for reproduction.2 The hamster should have an environmental temperature of 65° to 70° F (18.3° to 21.1° C) or 71° to 75° F (21.6° to 23.8° C) for young.3
NUTRITION
In the wild, hamsters as a group are mainly herbivorous but may also eat insects, lizards, frogs, mice, young birds, and snakes. The wild diet is comprised mainly of seeds, shoots, root vegetables, and leaves and flowers of items such as wheat, barley, millet, soybeans, peas, potatoes, carrots, and beets.1 Hamsters like to store food and bedding: A single hamster was found to have stored 90 kg of plant material.1 Smaller materials are carried in the cheek pouches, but large materials are carried with the incisors for storage in the burrow.
Wild gerbils are opportunistic vegetarians and eat primarily seeds, fruits, leaves, stems, and roots. However, they may also eat insects, snails, reptiles, and even other small rodents. Most gerbils take their food back to their burrow for storage before consuming it. One Mongolian jird was documented to have 20 kg of seeds stored in its burrow.6
Hamsters and gerbils have similar dietary requirements in captivity and should be given a diet with a minimum of 16% protein and 4% to 5% fat. However, gerbils may require dietary protein of 20% or more. Formulated diets in pellet and block form are recommended for both hamsters and gerbils. Occasional treats should be limited to those high in protein and low in fat. Seed-based diets should not be fed exclusively to hamsters, as they are prone to osteoporosis.8
Hamsters eat about 12 g of feed and 10 ml water for every 100 g of body weight daily and are coprophagic.3 Newborn hamsters begin eating on their own at 7 to 20 days of age. Hamsters should be fed from the floor rather from a hopper to avoid both lesions around the nares and neglect of pups. Gerbils eat about 8 g of feed per day. Young gerbils begin eating solid food at about 14 days of age. Because gerbils are physiologically adapted to arid regions, they only consume about 4 ml of water per day and produce very little urine.
RESTRAINT
Manual Restraint of Hamsters
Hamsters are agonistic in nature and may bite if roughly handled, startled, awakened, or injured.4 Do not surprise or startle the hamster before handling.2 Wake the hamster gently if it is sleeping, as they are deep sleepers.4 One effective method for restraint is to place the hamster on a flat surface and cover it with the palm of your hand, with your thumb near the hamster’s head.2 The loose skin over the back should be firmly grasped with all the fingers, lifting the hamster. If the skin is taut over the abdomen and chest, the hamster will be securely held, although the eyes may bulge with this over-the-back grip. Hamsters may also be picked up by the scruff over the dorsal cervical area and shoulders.4 When scruffing the hamster, beware of the extensive cheek pouches and excessive loose skin of this animal, which facilitate the hamster’s ability to turn and bite the handler.
Manual Restraint of Gerbils
Gerbils are generally docile animals, require minimal restraint, and can be picked up and loosely cupped in one hand (Figure 15-7).8 However, the excited, threatened gerbil will rhythmically thump its rear foot and may bite. Gerbils can be restrained similarly to the hamster, with back or scruff techniques. The initial capture of a gerbil may be facilitated by grasping the base of the tail (Figure 15-8). Tail degloving or detachment can occur as an antipredator mechanism and is more likely to occur when the tail is grabbed distally or the animal is young. Grasp the base of the tail with one hand and then use the forefinger and thumb to gently pin down the head. With the remaining fingers, grasp the loose skin along the neck and back and use the little finger to hold the tail (Figure 15-9). Alternately, the docile gerbil may be scooped up in the palm.
PERFORMING A PHYSICAL EXAMINATION
Any lesions of the skin and pelage should be noted and the lymph nodes and mammary glands palpated. Abdominal palpation may reveal masses, and fecal or urine staining may represent gastrointestinal disease, urinary tract infection, or reproductive tract disease. The plantar surface of each foot should be examined for pododermatitis and trauma.
Common abnormalities found on the physical examination include diseases affecting the eyes, and the integumentary, respiratory, and gastrointestinal systems.10 Common disease presentations that affect small pet rodents include trauma, coma, respiratory disease, skin scald, malocclusion, and diarrhea or other gastrointestinal disturbances.7
Diseases of the integument of hamsters and gerbils include ectoparasitism, endocrine disease, trauma, sore nose, neoplasias, and abscesses. Demodectic mange and other mites and lice cause hair loss in hamsters and gerbils. Symmetric hair loss of the flanks, hyperkeratosis, and hyperpigmentation of the skin are associated with adrenal disease in the hamster.10 Trauma from cagemates occurs both to hamsters and gerbils and involves the fur and skin, especially of the urogenital region in males.10 Gerbils can also develop “sore nose,” a facial dermatitis of unknown etiology. Neoplasias and abscesses affecting the skin may also be diagnosed.
Ophthalmologic diseases are common in hamsters and gerbils. Transient cataracts may develop secondary to anesthesia; ocular lubrication and closure of the eyelids during anesthetic episodes may limit this occurrence and prevent iatrogenic corneal injuries.10 Exophthalmos occurs in ham-sters and is due to trauma, infection, and inappropriate restraint. Correction of the initiating cause and the proptosis in a timely manner is required for a good prognosis with these cases.10
DIAGNOSTIC TESTING
Clinical Pathology
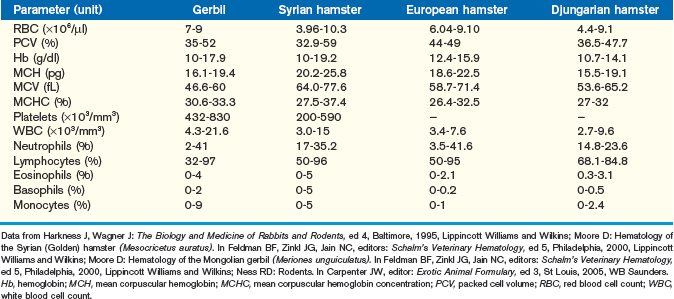
Excellent, detailed reviews of the hematology of the gerbil and hamster exist and should be consulted when more than a brief clinical summary, as provided here, is necessary for patient diagnosis (Tables 15-3 and 15-4).11–13
TABLE 15-4 Serum Biochemical Values of the Hamster and Gerbil
| Analyte (unit) | Gerbil | Syrian hamster |
|---|---|---|
| ALT (IU/L) | – | 22-128 |
| AP (IU/L) | – | 99-186 |
| AST (IU/L) | – | 28-122 |
| Bilirubin, total (mg/dl) | 0.2-0.6 | 0.1-0.9 |
| Glucose (mg/dl) | 50-135 | 37-198 |
| Calcium (mg/dl) | 3.7-6.2 | 5.3-12 |
| Phosphorous (mg/dl) | 3.7-7 | 3.0-9.9 |
| Creatinine (mg/dl) | 0.6-1.4 | 0.4-1 |
| Urea nitrogen (mg/dl) | 17-27 | 12-26 |
| Protein, total (g/dl) | 4.3-12.5 | 5.2-7.0 |
| Albumin (g/dl) | 1.8-5.5 | 3.5-4.9 |
| Globulin (g/dl) | 1.2-6.0 | 2.7-4.2 |
| Triglycerides | – | 72-227 |
| Cholesterol (mg/dl) | 90-150 | 55-181 |
| Sodium (mEq/L) | 141-172 | 128-144 |
| Potassium (mEq/L) | 3.3-6.3 | 3.9-5.5 |
Data from Harkness J, Wagner J: The Biology and Medicine of Rabbits and Rodents, ed 4, Baltimore, 1995, Lippincott Williams and Wilkins; Ness RD: Rodents. In Carpenter JW, editor: Exotic Animal Formulary, ed 3, St Louis, 2005, WB Saunders.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree