CHAPTER 17 GUINEA PIGS
COMMON SPECIES KEPT IN CAPTIVITY
Extensive breeding has resulted in numerous varieties of coat color and characteristics. The most common breeds are the American (or English) which has a short, smooth coat and the multicolored Teddy (Figure 17-1); Abyssinian (Figure 17-2), which has a medium length coat in a whorled pattern; and the Peruvian, which has a very long, smooth coat.
BIOLOGY
The dentition of the guinea pig is described as aradicular hypsodont (e.g., all teeth have a relatively long crown and are “open rooted”).1 The maxilla is slightly wider than the mandible, and the occlusal angle of the premolars and molars is marked compared to other rodent species. The dental formula of the guinea pig is 2(I 1/1, C 0/0, PM 1/1, M 3/3) = 20. The maxillary incisors are much shorter than those set in the mandible. The molars and premolars are not easily visualized without special instrumentation because of the small size of the oral cavity and tendency for the involution of the buccal surface.
Females are sexually mature at 6 weeks of age, whereas males on average reach puberty approximately 4 weeks later. Gestation is long, when compared to other rodents, at 68 days.2 As a result of this long gestation period, young are precocial when born. Juvenile pigs usually eat solid foods by 4 or 5 days of age.3 Litter sizes range from 1 to 6, with an average of 3 to 4 young.2 A female guinea pig should deliver her first young before she is 6 months of age. If birth has not occurred before 6 months of age, the pubic symphysis becomes mineralized, with future pregnancies resulting in an inability of the sow to naturally deliver the babies. Female guinea pigs that become pregnant after 6 months of age invariably require cesarean section deliveries.
HUSBANDRY
Housing
Guinea pigs are best housed in well-ventilated, wire-sided cages with solid bottoms. Wire-bottom cages may also be used; however, care must be taken to ensure that the mesh is small enough that a limb cannot become entrapped. An area of solid flooring should be provided, as uninterrupted time on wire mesh may predispose the guinea pig to pododermatitis. Adequate space is needed in the enclosure for the guinea pig to move about unencumbered with enough space for a hide box. Hide boxes or a secluded space is required for prey species (e.g., rodents) to reduce stress that may lead to disease problems. Substrate products that contain aromatic oils (e.g., cedar and pine shavings) should not be used, as they can act as contact and respiratory irritants. Appropriate bedding materials include recycled newspaper products, shredded paper, and aspen shavings. The enclosure should be cleaned thoroughly on a regular basis (e.g., 2 times per week) because unsanitary conditions predispose the guinea pig to pododermatitis, respiratory, and other health problems. If housed indoors, guinea pig enclosures do not require a cover, as these animals do not typically jump or climb. However, the sides of the enclosure should be high enough to prevent escape (approximately 25 cm).2 Heavy food containers are recommended to make dumping of the receptacle more difficult. All food containers should be easy to disinfect and cleaned regularly, as guinea pigs have a habit of soiling their food bowls. Most guinea pigs readily accept drinking water from a sipper bottle, which will decrease spillage and will keep feces, urine, and bedding from contaminating the water.
Diet
Because guinea pigs lack the enzyme L-gulonolactone oxidase, they are unable to synthesize ascorbic acid from glucose. Therefore, guinea pigs require supplemental vitamin C in their diets. Although commercial guinea pig pellets are manufactured with vitamin C, the supplement often degrades rapidly, especially if the pellets are subjected to high heat and humidity. Vitamin C placed in drinking water also degrades rapidly and should be changed daily. To ensure that a guinea pig is receiving a proper amount of vitamin C, it is necessary to supplement a diet of pellets and hay with plenty of fresh foods or often a specifically manufactured vitamin C supplement tablet (Oxbow, Inc., Murdock, NE). Many green, leafy vegetables, such as kale, mustard greens, dandelion greens, parsley, and many others, are excellent sources of ascorbic acid (Box 17-1). The vitamin C requirement of an adult, nonbreeding guinea pig is 10 mg/kg/day.2,3
BOX 17-1 Vitamin C content of selected fruits and vegetables
| Vegetables | mg vitamin C/100 g |
|---|---|
| Red bell pepper | 190 |
| Parsley | 133 |
| Kale | 120 |
| Broccoli | 93.2 |
| Brussels sprouts | 85 |
| Mustard greens | 70 |
| Turnip greens | 60 |
| Cauliflower | 46.4 |
| Dandelion greens | 35 |
| Cilantro | 27 |
| Romaine lettuce | 24 |
| Sweet potato | 22.7 |
| Carrot | 9.3 |
| Fruits | mg vitamin C/100 g |
|---|---|
| Kiwi | 98 |
| Strawberry | 56.7 |
| Orange | 53.2 |
| Cantaloupe | 42.2 |
| Apple | 5.7 |
PREVENTIVE MEDICINE
Many health problems of guinea pigs are related to improper husbandry. During a routine veterinary visit, the owners should be asked to provide a detailed description of the animal’s housing environment, including substrate, frequency of cleaning, ambient temperature, and exercise time. The diet history is also important. Owners should be asked not only what the guinea pig is offered, but also in what proportions and of what foods the animal actually eats.
RESTRAINT
Most guinea pigs are quite docile and do not require aggressive restraint. Often a hand on the animal’s dorsum is adequate to restrain a guinea pig patient on the examination table (Figure 17-3). When transporting a guinea pig, support the body with one hand under the thorax and abdomen while placing the other hand on the back to prevent the patient from falling or jumping (Figure 17-4).
PERFORMING A PHYSICAL EXAMINATION
Obtaining an accurate body temperature, heart rate, and respiratory rate is best accomplished at the beginning of the examination, as these parameters will invariably change with handling. An accurate weight should be obtained using an electronic gram scale. A “hands-on” physical examination should begin with the head; the veterinarian should assess the eyes for symmetry or discharge and check that the external pinnae of the guinea pig are hairless, as normal. The external ear canals often contain a small to moderate amount of dark, ceruminous debris. The nasal planum should be dry and flat, whereas palpation of the ventral mandible may reveal deformities secondary to overgrowth of molar and premolar apices.
The guinea pig coat varies somewhat with breed but, in general, should be smooth and shiny. Guinea pigs often have a mild to moderate amount of dark sebaceous debris on the skin of the dorsum. Older male guinea pigs may develop a focal accumulation of this debris at the base of the vertebral column, which may be referred to as the “grease gland.”4
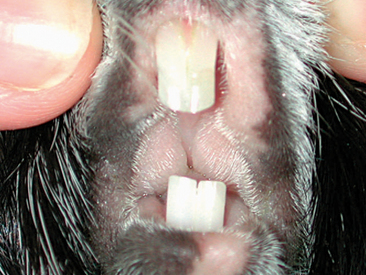
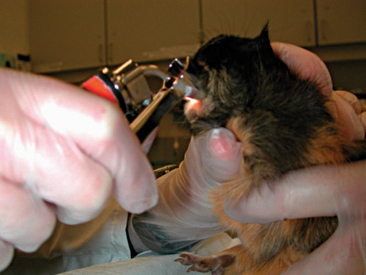
A complete oral examination is an important part of the guinea pig exam. Because of the potential stress associated with the oral exam, this evaluation should be reserved for the end. The oral cavity of the guinea pig is very narrow with a small opening, making visualization difficult (Figure 17-5). Instruments such as an otoscope with cone or a human nasal speculum will increase visualization of the caudal oral cavity (Figure 17-6). However, many dental lesions may be overlooked using these methods, and anesthesia is often required to adequately determine oral health.
DIAGNOSTIC TESTING
Venipuncture
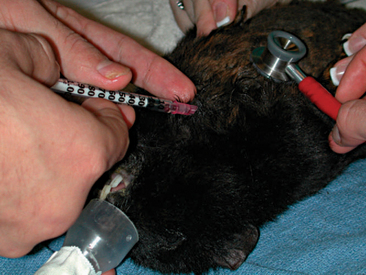
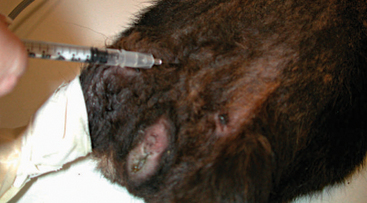
Obtaining blood samples from guinea pigs for routine diagnostics is a challenging procedure. Peripheral venipuncture sites available for most mammals (e.g., lateral saphenous vein and cephalic vein) are difficult to visualize in guinea pigs and only allow for the collection of small sample volumes. The jugular vein may be used; however, the short neck of the guinea pig and poor tolerance of the aggressive restraint by the patient restrict the availability of this site for blood collection. Often to obtain an adequate blood sample, it is necessary to anesthetize the patient. Once anesthetized, the large central vessels may be accessed with less stress on the patient and phlebotomist. Acceptable venipuncture sites while the patient is anesthetized include the cranial vena cava (Figure 17-7), femoral vein (Figure 17-8), and jugular vein.
Hematology
Baseline blood work is an important tool in the routine monitoring of health as well as in the diagnosis of disease. A complete blood count is essential for assessing red blood cell and white blood cell parameters (Box 17-2).
BOX 17-2 Hematologic parameters for guinea pigs
Data from Quesenberry KE, Donnelly TM, Hillyer EV: Biology, husbandry, and clinical techniques. In Quesenberry KE, Carpenter JW, editors: Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery, ed 2, St Louis, 2003, WB Saunders; Carpenter JW: Hematologic and serum biochemical values of rodents. In Carpenter JW, editor: Exotic Animal Formulary, ed 3, St Louis, 2005, WB Saunders; Campbell TW: Mammalian hematology: laboratory animals and miscellaneous species. In Thrall MA, editor: Veterinary Hematology and Clinical Chemistry, Baltimore, 2004, Lippincott Williams and Wilkins.
| Hematologic Parameter | Reference Interval |
|---|---|
| PCV (%) | 30-50 |
| RBC (×106 cells/μl) | 4-11 |
| Hb (g/dl) | 11-17 |
| MCV (fl) | 70-95 |
| MCHC (%) | 25-40 |
| MCH (pg) | 23-27 |
| WBC (×103 cells/μl) | 6-17 |
| Heterophils (%) | 20-60 |
| Lymphocytes (%) | 30-80 |
| Monocytes (%) | 1-10 |
| Eosinophils (%) | 0-7 |
| Basophils (%) | 0-3 |
| Platelets (×103 cells/μl) | 300-600 |
Guinea pigs have heterophils rather than neutrophils as the predominant circulating granulocyte. Heterophils lack myeloperoxidase, the enzyme that causes purulent, liquid exudates. Therefore, the debris contained in guinea pig abscesses is often found to be very thick and caseous, a fact that must be understood when treating these conditions. A normal guinea pig white blood cell differential will consist of primarily heterophils and lymphocytes, usually with a greater proportion of lymphocytes. The remaining leukocyte types (e.g., monocytes, eosinophils, basophils) are normally present in very low numbers. Early stages of a guinea pig’s inflammatory response are often characterized by a shift in the differential white cell ratio (e.g., increased heterophils, decreased lymphocytes) rather than an increase in total leukocyte count. This ratio shift makes evaluating the entire leukogram essential for health evaluation (Box 17-3). The platelet count is also an important marker of inflammation in guinea pigs and other small mammal species. Large increases in the platelet count (>1,000,000/ml) can be seen without an increase in the total white blood cell count.
BOX 17-3 Example of an inflammatory leukogram in a guinea pig
| Hematologic Parameter | Value |
|---|---|
| HCT (%) | 43 |
| WBC (×103 cells/μl) | 13.1 |
| Heterophils (×103 cells/μl/%) | 7.8/59.3 |
| Lymphocytes (×103 cells/μl/%) | 3.4/25.9 |
| Monocytes (×103 cells/μl/%) | 1.2/8.8 |
| Eosinophils (×103 cells/μl/%) | 0.7/5.4 |
| Basophils (×103 cells/μl/%) | 0.09/0.7 |
| Platelets (×103 cells/μl) | 694 |
A cell type that is unique to guinea pigs is the Kurloff cell. These large lymphocytes contain a cytoplasmic inclusion (e.g., a Kurloff body). Kurloff cells are noted most often in the peripheral blood of reproductive age females, although they are also identified in male guinea pigs. The number of circulating Kurloff cells will increase in response to exogenous estradiol and testosterone administration, although the effects were more dramatic with estradiol administration.7,8 Other studies have shown the disappearance of Kurloff cells in spayed female and castrated male guinea pigs.8 The function of the Kurloff cell is not completely understood, and these cells appear to lack lysosomes. At this time, there is no evidence that the Kurloff cell has phagocytic activity.8 The activity of Kurloff cells appears to most closely correlate with that of natural killer cells found in other species.7
Clinical Chemistries
Plasma biochemical analysis is necessary for evaluation of organ function as well as plasma glucose and proteins. It is important to interpret the results of a chemistry panel in conjunction with history, physical exam findings, and the results of other diagnostic tests. Reference intervals for biochemical parameters are shown in Box 17-4.
BOX 17-4 Plasma biochemical parameters for guinea pigs
Data from Quesenberry KE, Donnelly TM, Hillyer EV: Biology, husbandry, and clinical techniques. In Quesenberry KE, Carpenter JW, editors: Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery, ed 2, St Louis, 2003, WB Saunders; Carpenter JW: Hematologic and serum biochemical values of rodents. In Carpenter JW, editor: Exotic Animal Formulary, ed 3, St Louis, 2005, WB Saunders; Campbell TW: Mammalian hematology: laboratory animals and miscellaneous species. In Thrall MA, editor: Veterinary Hematology and Clinical Chemistry, Baltimore, 2004, Lippincott Williams and Wilkins.
| Biochemical Parameter | Reference Interval |
|---|---|
| Sodium (mEq/L) | 120-150 |
| Potassium (mEq/L) | 4-8 |
| Chloride (mEq/L) | 90-115 |
| Glucose (mg/dl) | 60-125 |
| Blood urea nitrogen (mg/dl) | 9-32 |
| Creatinine (mg/dl) | 0.6-2.2 |
| Calcium (mg/dl) | 8-12 |
| Phosphorous (mg/dl) | 3-8 |
| Total protein (g/dl) | 4-7 |
| Albumin (g/dl) | 2-5 |
| Globulin (g/dl) | 2-4 |
| Creatine kinase (IU/L) | 0-300 |
| Aspartate transferase (IU/L) | 30-70 |
| Alkaline phosphatase (IU/L) | 60-110 |
| Bilirubin (mg/dl) | 0-1 |
| Cholesterol (mg/dl) | 20-50 |
Urinalysis
Urinalysis is a useful diagnostic test in guinea pigs with signs of upper or lower urinary tract disease. Urine samples may be obtained by free catch, floor catch, or cystocentesis. If urine is to be cultured, it is ideal to use the cystocentesis method of collection. Ultrasound guidance and/or mild sedation of the patient will aid the veterinarian and/or veterinary technician in obtaining the urine sample.
Imaging
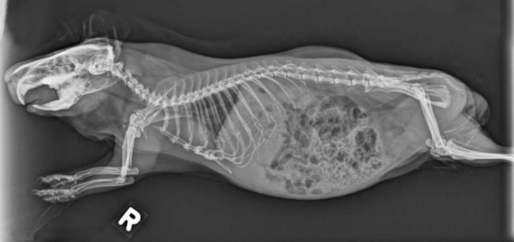
Whole body radiographs may provide a large amount of information for the veterinarian treating an ill patient. Both lateral and ventrodorsal (or dorsoventral) views should be obtained (Figures 17-9 and 17-10). To minimize rotation, care should be taken to extend the limbs symmetrically when positioning the patient. Because guinea pigs have stocky builds with short limbs, and because they resent aggressive restraint, sedation or anesthesia is helpful in obtaining diagnostic radiographs as well as in reducing the patient’s stress (Figure 17-11). Table 17-1 is a guideline for radiographic techniques used in common small mammal radiographic studies.
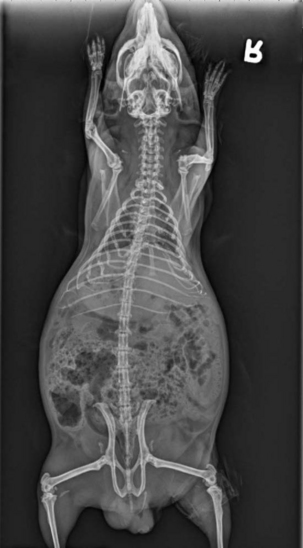
Figure 17-10 Ventrodorsal radiograph of the same guinea pig pictured in Figure 17-9.
(Courtesy University of California–Davis.)
TABLE 17-1 Guidelines for Radiographic Techniques for Selected Radiographic Studies in Guinea Pigs
| Anatomic location | mA | kVp |
|---|---|---|
| Whole body | 5.0 | 44 |
| Extremities | 7.5 | 54 |
| Skull | 6.0 | 48-53 |
From Silverman S, Tell LA: Radiology equipment and positioning techniques. In Silverman S, Tell LA, editors: Radiology of Rodents, Rabbits, and Ferrets: An Atlas of Normal Anatomy and Positioning, St Louis, 2006, WB Saunders. kVp, kilovolt peak; mA, milliampere.
Ultrasound is another imaging modality that is very useful in the diagnosis of common guinea pig disease processes, such as ovarian cysts (Figure 17-12) and urinary tract calculi. As with radiographs, sedation or anesthesia can assist in reducing patient stress and improve the quality of images. Guinea pigs often have a large amount of gas accumulation in the GI tract, which obscures the ultrasound image, sometimes making this imaging technique of limited value.
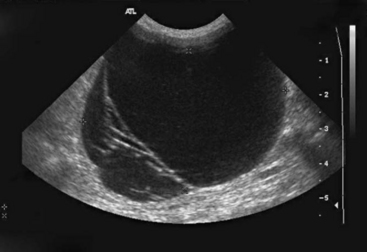
Figure 17-12 Ultrasound image of a large ovarian cyst in a mature female guinea pig.
(Courtesy University of California–Davis.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree