Fig. 2.1.
Spontaneous locomotor activity in prenatal E17 MAM- vs. saline-exposed rats. Total distance (±SEM) travelled by sham and MAM-exposed rats during a 60-min testing period in the open field was measured at pre- and post-puberty. *** p < 0.001 vs. sham. (Adapted from Le Pen et al., 2006)
In contrast to these results, data obtained in Wistar rats with 22 mg/kg E17 MAM exposure are more inconsistent. Indeed, in a large open field, Flagstad et al. (32) did not observe any modification of locomotor activity level in MAM-exposed rats in comparison to sham animals, whereas, in the same arena, MAM-exposed rats exhibited increased spontaneous locomotor activity when tested in the social interaction test. These discrepancies could be due to a different strain of rats or to the experimental conditions (lighting during light (Sprague Dawley rats) vs. dark (Wistar rats) period of the light-dark cycle, MAM dose).
2.1.2 Dopaminergic Receptor Agonist-Induced Hyperactivity
Two different research groups have conducted experiments to investigate amphetamine-induced hyperactivity in animals pre-exposed to MAM (22 mg/kg) at E17 (32, 36) during the dark period of the light-dark cycle. These two studies performed in two different strains of rats (Wistar and Fisher 344) both revealed that MAM-exposed rats were hypersensitive to the locomotor-activating effects of d-amphetamine (Wistar: 2 mg/kg s.c. measured as salt 90 min and Fisher 344: 0.5 mg/kg i.p. 60 min). In addition, distinct experiments performed before and after puberty (Fig. 2.2) showed that hypersensitivity to amphetamine emerged only in adult animals (36).
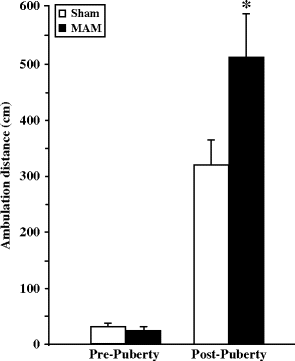

Fig. 2.2.
Amphetamine-evoked locomotor activity in prenatal E17 MAM- vs. saline-exposed rats. Total distance (±SEM) travelled by sham and MAM-exposed rats during a 60-min testing period in the open field after amphetamine (0.5 mg/kg, i.p.) was measured at pre- and post-puberty. * p < 0.05 vs. sham. (Adapted from Moore et al., 2006)
2.1.3 Glutamatergic NMDA Antagonist-Induced Hyperactivity and Stereotyped Behaviors
PCP
Hypersensitivity to PCP-induced hyperlocomotion in an open field has been shown at adulthood, in two strains of rats (Wistar and Fisher 344) pre-exposed to MAM (22 mg/kg) (36, 37) after administration of an acute s.c. (120 min) (Fig. 2.3) or i.p. (60 min) dose of 5 mg/kg. In addition, it has been shown that the increase of PCP-induced ataxia in the open field was similar in sham and MAM-exposed rats (Fig. 2.4) (36). Orofacial dyskinesia and stereotypies (vacuous chewing and biting stereotypies) induced either spontaneously or by PCP (2.5 and 5 mg/kg i.p.) were also investigated in adult MAM-exposed rats (36). Oral behaviors were manually scored by direct observation (by an observer blind to gestational and drug conditions) and defined as follows: tremor, rapid oscillation of facial muscles progressing from lower jaw through cheek to extra-ocular region, stereotypic mouthing of the tail, and repetitive approaches to the tail with the mouth featuring horizontal and vertical motions of the jaw (follow-through biting rarely occurred). These behaviors were scored every 5 min during a 60-min period as absent [0], present/intermittent [1], or continuous [2]. MAM-exposed animals displayed a significantly higher frequency of spontaneous orofacial dyskinesias and stereotypies; moreover, these behaviors were significantly more sensitive to exacerbation by PCP (Fig. 2.5) (36).
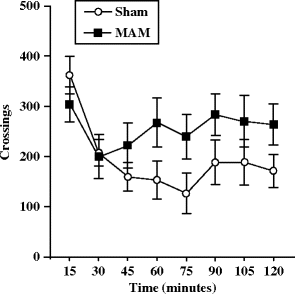
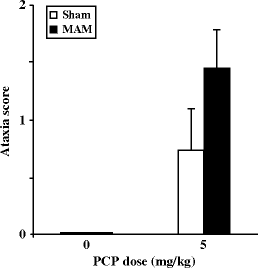
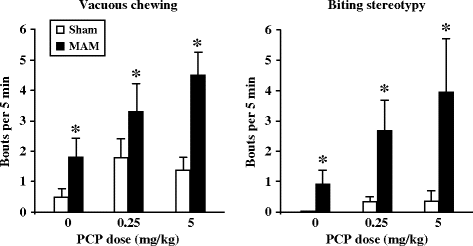

Fig. 2.3.
Phencyclidine-evoked locomotor activity in prenatal E17 MAM- vs. saline-exposed rats. Total distance (±SEM) travelled by sham and MAM-exposed rats during a 120-min testing period in the open field after phencyclidine (5 mg/kg, s.c.) was measured at adulthood. (Adapted from Penschuck et al., 2006)

Fig. 2.4.
Phencyclidine-evoked ataxia in prenatal E17 MAM- vs. saline-exposed rats. Ataxia scores were measured in sham and MAM-exposed adult rats during a 60-min testing period after phencyclidine (5 mg/kg, i.p.) at adulthood. (Adapted from Moore et al., 2006)

Fig. 2.5.
Phencyclidine-evoked orofacial dyskinesias in prenatal E17 MAM- vs. saline-exposed rats. Vacuous chewing (left panel) and biting stereotypy (right panel) were measured following injection of saline or phencyclidine (PCP 2.5, 5.0 mg/kg, i.p.) at adulthood. * p < 0.05 vs. sham. (Adapted from Moore et al., 2006)
MK-801
The sensitivity to (+)-MK-801 (0.05 and 0.1 mg/kg) has been investigated in E17 MAM-exposed (25 mg/kg) Sprague Dawley rats at both pre- and post-puberty in different groups of rats (PD35 and PD115, respectively) to avoid multi-drug exposure (Fig. 2.6). Before MK-801 testing, animals were subjected to a 1-h session of habituation in the locomotor activity apparatus (open field: 40 cm × 40 cm), followed by another 1-h period of locomotor activity after a saline injection. Whereas both sham and MAM-exposed rats exhibited a similar response to the locomotor-activating effects of MK-801 before puberty, MAM-exposed rats developed hypersensitivity to the locomotor-activating effect of MK-801 in adulthood (35).
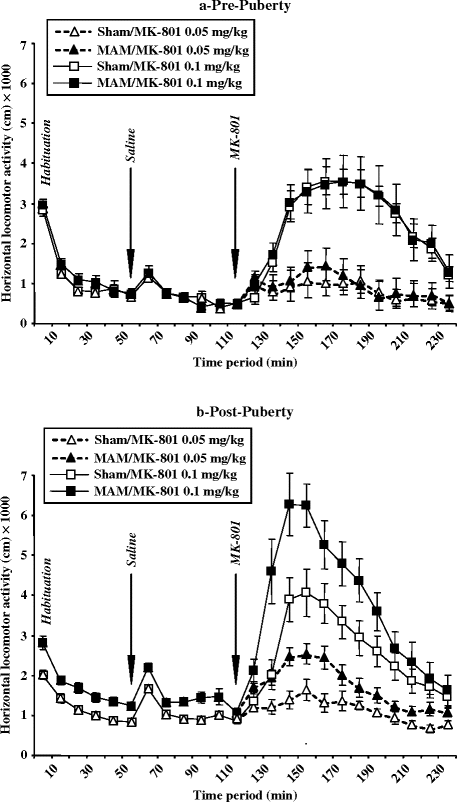

Fig. 2.6.
(+) MK-801-evoked locomotor activity in prenatal E17 MAM- vs. saline-exposed rats. The distance (±SEM) travelled by sham and MAM-exposed rats was explored at both (a) pre- and (b) post-puberty in a spontaneous exploration session, after saline injection and after (+)-MK-801 (0.05 or 0.1 mg/kg s.c.) administration. (Adapted from Le pen et al., 2006)
2.2 Behavioral Anomalies Reminiscent of Negative Symptoms of Schizophrenia
Social withdrawal is a frequent negative symptom of schizophrenia (1, 3). It exists prior to the onset of disease and is predictive of future schizophrenia (57). In rodents, social behavior can be easily evaluated using a social interaction test. This test consists in allowing the experimental subject to freely explore an unfamiliar congener in its home cage or in a neutral environment. Social exploration is measured in time spent by the experimental subject around the congener.
2.2.1 Procedure
The general design of the social interaction test used to evaluate MAM-exposed animals was adapted from the work of Sams-Dodd (58). The test was performed in an open arena (80 cm × 80 cm, 50 cm high) that was placed in a dimly lit room. Two unfamiliar rats that had approximately the same weight and had received identical prenatal treatment were placed simultaneously in the opposite corners of the arena and the track of each rat was recorded for a 10-min period with a video tracking system. Social interactions were measured by the time spent and number of contacts at a distance lower than 20 cm from each other. During the 10-min period of the test, locomotor activity and time spent in the central zone were also recorded.
2.2.2 Results
In Wistar and Sprague Dawley rats tested during the dark and light phase of the light-dark cycle, respectively, evaluation of social behavior produced similar results. Indeed, deficits in social interaction revealed by a decreased amount of time spent in contact with the congener were first shown in adult Wistar rats exposed to MAM at E17 (32). We extended these results (Fig. 2.7) by showing that Sprague Dawley MAM-exposed rats display these deficits at both pre-puberty and adulthood (34, 35). The deficits could be dissociated from motor performances, given that hyperactivity emerged only after puberty (34, 35) and were not the result of anxiogenic-like behaviors since the time spent in the central zone was similar in both groups (32, 34, 35). In addition, this abnormal behavior appeared to be the consequence of a lower duration of contacts and not a difference in the number of contacts (32, 34, 35).
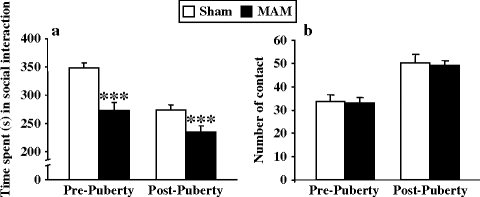

Fig. 2.7.
Social interactions in prenatal E17 MAM- vs. saline-exposed rats. The time spent (±SEM) in interaction (a) and the number of contacts (b) between two rats from the same treatment group were explored at pre- and post-puberty. *** p < 0.001 vs. sham. (Adapted from Le pen et al., 2006)
2.3 Abnormalities Reminiscent of the Cognitive Symptoms of Schizophrenia
Cognitive impairments constitute a central feature of schizophrenia (59) that include deficits in abstraction, executive function, verbal memory, language function, vigilance, and attention (60–62). Schizophrenic patients are also commonly described with abnormalities in information processing (63–65). For some authors, sensorimotor gating deficits correlate highly with measures of perceptual and reasoning disturbances. This relationship may then form an important basis for cognitive dysfunction observed among schizophrenic patients (66). Thus, an important future direction of preclinical research relating to schizophrenia is the design of animal models and novel treatments that target cognitive dysfunctions associated with this disorder. It is possible to design behavioral paradigms in rodents that can evaluate cognitive constructs “comparable” with those measured in many human experimental paradigms.
2.3.1 Working Memory
Working memory may be defined as the capacity to hold information online over short delays while that information is integrated with other ongoing mental operations. A number of studies have shown that schizophrenic patients have deficient working memory (67–69), and imaging studies have confirmed that these deficits in working memory are associated with abnormal activation of the prefrontal cortex (70). Although working memory as measured in rodent might differ from that in human because it is explored for a longer delay, performance in working memory has been investigated in MAM-exposed rats using various paradigms.
Radial Arm Maze
In the rat, interactive communication between the hippocampus and the medial prefrontal cortex is necessary to perform a spatial working memory task. Thus, a delay-interposed radial maze learning task, previously shown to be mediated by a distributed neural network linking the hippocampus and the prefrontal cortex (71), has been used to investigate working memory in MAM-exposed rats (33). The radial arm maze task takes advantage of the natural tendency of food-deprived rodents to learn and remember different spatial locations for food in an eight-arm radial maze.
Procedure
The eight-arm radial maze used to test working memory in MAM-exposed rats consisted of a central arena (30 cm diameter) connected to eight equally spaced arms (80 cm × 13 cm) that extended radially. The maze was elevated 60 cm above the floor and surrounded by extra-maze three-dimensional cues in an illuminated room, with music on for background noise. The working memory task was adapted from Packard et al. (72) and Floresco et al. (73). After a 3-day period of habituation to the maze environment, adult food-deprived animals were trained. Each trial consisted of a training and a test phase, separated by a delay. Every day, a random set of four arms (the same for all animals in a given day) was blocked. Food pellets were placed in cups at the end of the remaining four open arms. Each rat was given 5 min to retrieve the pellets and then was replaced in its home cage for the delay period. Then, during the test phase, all arms were open, but food was placed only in the arms previously blocked. Rats were allowed a maximum of 5 min to retrieve the four pellets (Fig. 2.8). The initial delay between training and test phases was 5 min. The learning curves (5-min trials) were compared two by two between groups by means of the log rank test. After achieving criterion performance (i.e., all four pellets retrieved in four or five choices during the test phase for 2 consecutive days), the delay was increased to 30 min. On 30-min delay test days, we recorded the number and order of arm entries. Entries into non-baited arms during the test phase were considered as errors. We distinguished across-phase errors (entries into an arm entered previously during the training phase) from within-phase errors (re-entries into an arm entered earlier during the test phase).
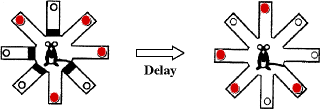

Fig. 2.8.
Diagram of the radial arm maze task with delay. Food pellets (plain circle) were placed in a well at the tip of each maze arm. (Adapted from Gourevitch et al., 2004)
Results
E17 MAM-exposed rats were able to reach the level of performance of control rats at the initial stages of training as revealed by similar number of 5-min trials necessary to learn the rule in both sham and MAM-exposed animals. Nevertheless, with a 30-min delay, MAM-exposed rats were unable to process and retrieve spatial information when a 30-min delay was interposed, indicating a significant impairment in working memory. Indeed, MAM-exposed rats made more total errors than did sham animals (Fig. 2.9). These errors were mostly related to working memory (entries during the test phase to an arm entered previously during the training phase), while errors related to visual short-term memory (re-entries into an arm entered earlier during the test phase) were similar in MAM-exposed rats compared to sham animals.
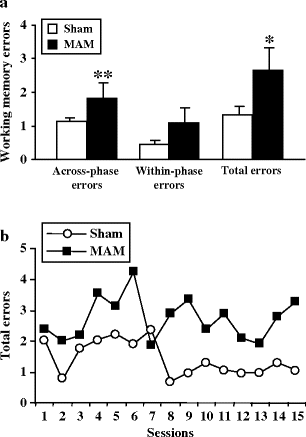

Fig. 2.9.
Working memory performance in a delay-interposed, eight-arm radial maze task in prenatal E17 MAM- vs. saline-exposed rats. (a) E17 MAM-exposed animals made more total errors than did controls, and (b) these errors were mostly across-phase errors, whereas within-phase errors were not significantly different in treated and control rats (* p < 0.05; ** p < 0.01). (Adapted from Gourevitch et al., 2004)
Spontaneous Alternation
The Y-maze spontaneous alternation procedure used to assess spatial working memory in MAM-exposed rats (34) is based on their natural tendency to explore novel environment. When placed in the Y maze, normal rats prefer to explore the least recently visited arm and thus tend to alternate visits between the three arms. To explore the three arms successively, the rat must maintain an ongoing record of most recently visited arms and continuously update such records. A rodent with impaired working memory cannot remember which arm it just visited and thus shows decreased spontaneous alternation (74, 75).
Procedure
The Y maze used for MAM-exposed rats has three identical arms (40 cm × 9 cm × 15 cm with walls 30 cm high) placed at 120° from each other. Numerous visual cues (geometrical forms) were placed on the ceiling and kept constant during the experiments. Rats were placed at the end of one arm of the Y maze and the sequence of arm entries was recorded during 10 min. The introduction arm was randomly assigned for each rat. An arm visit was recorded when a rat moved all four paws into the arm. An alternation was defined as consecutive entries into all three arms (e.g., 1, 2, 3 or 1, 3, 2). The number of maximum alternations was the total number of arm entries minus 2 and the percentage of alternations was calculated as the ratio of actual to maximum alternations multiplied by 100: (actual alternations/maximum alternations) × 100. Persevering behavior was defined as subjects making significantly fewer alternations than would be expected by chance (50%).
Results
Deficits in alternation were present at both pre- and post-puberty in MAM-exposed rats compared to sham animals (Fig. 2.10). There was an improvement across puberty in both groups. Low performances before puberty in both sham and MAM-exposed rats were linked to a low exploratory behavior that could reflect anxiety-like behaviors in both sham and MAM-exposed young rats (34). The deficit in alternation indicates a deficit in flexibility and short-term memory, further supporting that E17 MAM exposure impairs the hippocampo-prefrontal networks (76). Nevertheless, spontaneous alternation results also suggest that the working memory skills used in the spatial alternation test were acquired during puberty and that maturation of processes involved in that behavior is not affected by the MAM treatment, since the deficits in MAM-exposed rats are already seen before puberty (34).
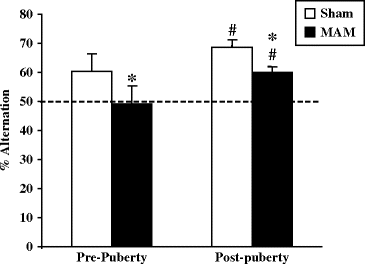

Fig. 2.10.
Spontaneous alternation in prenatal E17 MAM-exposed vs. saline-exposed rats. Percentage of alternation (±SEM) at pre- and post-puberty. The dotted line represents the chance level (50%), # different from the chance level p < 0.001. * p < 0.05 vs. sham group.
Delayed Non-match to Position
The delayed non-match-to-position task measures the ability of subjects to learn a rule in which they have to associate the position of a stimulus previously presented and an action for getting a reward. Flagstad and colleagues (31) have investigated working memory in MAM-exposed rats using this paradigm.
Procedure
In the procedure used by Flagstad and colleagues (31), in the first step, rats have to learn to press a lever to initiate the delivery of water (here rats were water deprived and water was used as the positive reinforcer). In the second step, the non-match-to-position (NMTP) training schedule, at the start of each trial, one of the two retractable levers is presented to the subject into the operant chamber. The subject has to press the lever for indicating that the sample has been registered. Then both levers are presented and the subject has to press the sample lever at the opposite of the one presented before when the rat received the reward. In the last step, the procedure was as in the NMTP experiment, except that a delay (0, 5, or 10 s, random) was inserted after the initial press on the right or left lever before the reward was given. The animals were initially trained for 25 sessions in this procedure including the delay and the subsequent three sessions were used as the test sessions.
Results
The investigation of working memory using the NMTP paradigm showed no consistent difference between the performances of the sham and MAM-exposed rats, either in the non-delay condition or in the delay condition (Fig. 2.11). The sham animals were superior only in four sessions out of 40 during the learning performance of the NMTP paradigm, and this did not appear to be a consistent effect (31).
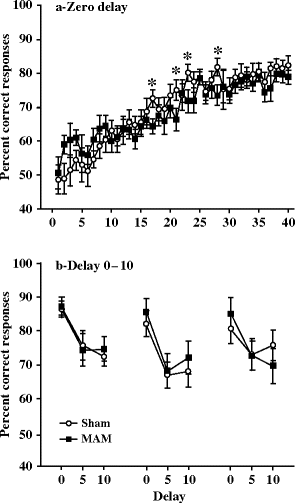

Fig. 2.11.
Working memory performance in a delay non-match-to-position (NMTP) task in prenatal E17 MAM- vs. saline-exposed rats. (a) The mean percent correct responses (±SEM) of the groups during the 40 days of acquisition of the NMTP paradigm. * p < 0.05 vs. sham. (b) The mean percent correct responses (±SEM) during three consecutive days of DNMTP. (Adapted from Flagstad et al., 2004)
In the delayed non-match-to-position procedure, a delay-dependent decline was apparent, but no differences between groups were observed. The accuracy of the performance in the task is believed to be dependent on prefrontal cortical integrity, as lesions decrease performance in the task, although the published data suggest that the deficits of the prefrontal cortex-lesioned rats are apparent only at the beginning of training (77, 78).
In Flagstad’s work (31), the animals were first trained on zero delay and then after an extensive training switched to the delay condition. The authors hypothesized that under the present protocol, the animals use a movement-mediated strategy, that is, the choice of the rat after the delay is mediated from the body position of the animal rather than by information kept online in working memory. Thus, this could possibly explain the lack of effect of the MAM treatment. During development, passively holding information available in working memory as well as recognition and recall from long-term memory is developed before active use and manipulations of information (executive functions), and as such not as dependent on later maturation of the prefrontal cortex (79). This might suggest that disturbances in prefrontal functions in MAM-exposed rats are related to a later maturation of this structure, and hence less radical than a dysfunction of all processes governed by the prefrontal cortex.
Morris Water Maze
Short-term place memory, or spatial working memory, has been shown to be impaired in schizophrenia (80, 81). In rodents, a typical experimental working memory paradigm is the Morris water maze (MWM) in which rats search for a submerged platform whose position is changed daily in a circular pool filled with water (82). The water maze is a particularly useful tool for assessment of spatial memory ability in rats. Indeed, the motivating stimulus, escape from water, does not require the food or water deprivation that is common in appetitive tasks like the radial arm maze or T maze. Flagstad and colleagues (31) have used the Morris water maze procedure to evaluate spatial working memory in MAM-exposed rats.
Procedure
The water maze consisted of a circular, black pool filled with water. The test room contained several permanent extra-maze cues such as the rat housing rack, laboratory table, posters on the walls, etc. In the working memory task, the platform was placed in a different quadrant every day (north-south-west-east) for 4 days. Before each trial, the rat was placed on the hidden platform for 15 s (inter-trial period). The initial placement on the platform each day served to reveal the new location of the platform. If the rat did not locate the platform within 60 s, the rat was gently placed on the platform. Key measures were path length (m), escape latency (s) to find the hidden platform, percent of path length near (10 cm) the sidewall (percent sidewall), and number of animals not finding the platform within 60 s (non-finders). These measures were recorded using a video tracking system.
Results
Flagstad et al. (31) showed that MAM-exposed rats performed significantly worse than controls on days 2 and 3 of testing but only on the basis of the percent sidewall and non-finder parameters. On the last day, however, they performed as well as the controls (Fig. 2.12). They concluded that the MAM treatment does not interfere with spatial working memory performance. Nevertheless, this result has to be confirmed since sham animals strangely performed worse on the last day of the test than on the penultimate day. The MAM treatment seems, however, to affect the search strategy of the rats. When the platform is moved, the rats spend more time swimming along the sidewall, rather than searching in the central part of the maze where the platform is positioned.
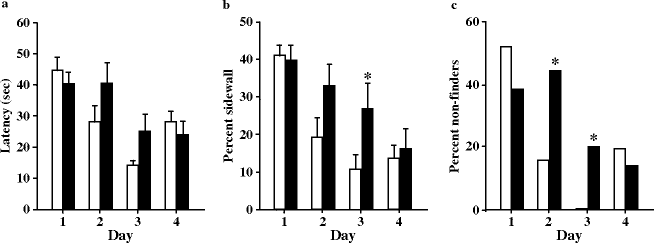

Fig. 2.12.
Working memory performance in the reversal learning paradigm of the Morris water maze in prenatal E17 MAM- vs. saline-exposed rats. The mean (±SEM) of latency to reach the hidden platform (a), percent of path length near (10 cm) the sidewall (b), and number of animals not finding the platform within 60 s (c) were presented for four consecutive trials. * p < 0.05 vs. sham. (Adapted from Flagstad et al., 2004)
2.3.2 Attention and Information Processing
Deficits in attention and information processing have been considered a central feature in schizophrenia, which might lead to stimulus overload, cognitive fragmentation, and thought disorder (65, 66, 83–88). Various physiological and neuropsychological techniques related to selective attention and pre-attentional mechanisms have been used to quantify information processing deficits in schizophrenia such as P50 (89, 90), prepulse inhibition (PPI) (83), latent inhibition (LI) (91, 92), continuous performance test (93, 94), span of apprehension (95, 96), visual backward masking (97, 98), and dichotic listening (99, 100). The advantage of PPI (101) and LI is that they can be used in both a human and an animal experimental setup. Whereas PPI assesses early attentional gating mechanisms, often referred to as pre-attentional deficits, LI assesses later stages of information processing related to attentional filtering. In addition, in rats, the five-choice serial reaction time task (5-CSRTT) developed by Carli and colleagues (102) exhibits closed analogies with the continuous performance test and Leonard’s 5-CSRTT used in humans to assess the processes involved in sustained attention (103, 104). Finally, attentional set-shifting task is considered as a rodent analog of the Wisconsin card sort task. PPI, LI, 5-CSRTT as well as attentional set-shifting tasks have been used to clarify attention and information processing in MAM-exposed rats (30, 31, 34, 36).
Prepulse Inhibition (PPI) of Startle Reflex
A well-established method for evaluating sensory filtering is the paradigm of PPI which refers to the inhibition of a startle reflex by presentation of a weak intensity prepulse immediately before the startle stimulus. The impact of MAM exposure on PPI has been investigated using two different protocols (34, 36).
Procedures
Acoustic startle reactivity and prepulse inhibition of startle reflex are assessed in a single session using standard startle chambers (SR-Lab, San Diego Instruments).
(A)
In Sprague Dawley rats (34, 35), after a 5-min acclimatization period (68 dB background noise), 10 startle pulses (120 dB, 40 ms duration) were presented with an average inter-trial interval of 15 s. During the next 20 min, no stimulus (background noise, 68 dB), prepulses alone (72, 76, 80, or 84 dB, 20 ms duration), startle pulses alone, six times for each condition, were randomly distributed.
(B)
In Fischer 344 rats (36), after a 5-min acclimatization period (55 dB background noise), in a first experiment, the startle threshold of both sham and MAM-exposed rats was determined with a repeated ascending, then randomized, series of 40-ms noise bursts ranging from 70 to 105 dB and in a second experiment, for PPI evaluation, rats were exposed to 100 dB startle tone (white noise burst), and 62 and 70 dB prepulses (6 or 12 ms duration). Prepulses were presented on 75% of the startle trials.
PPI was defined as the percent reduction in startle amplitude in the presence of the prepulse compared with the amplitude in the absence of the prepulse [100 × (startle amplitude on prepulse−pulse trials/startle amplitude on pulse-alone trials) × 100] (Fig. 2.13).
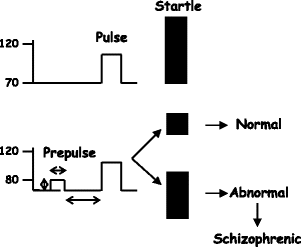

Fig. 2.13.
Scheme of prepulse inhibition of startle reflex response.
Results
PPI experiments in both Sprague Dawley and Fisher 344 rats revealed that E17 MAM-exposed animals at adulthood exhibited sensorimotor gating deficits (34, 36). In addition, we showed that these deficits emerge only after puberty (Fig. 2.14) (35). Besides, Moore and colleagues (36) showed that the PPI deficits were neither due to a decrease in sensory processing, since MAM-exposed rats showed a lower threshold for responding to startle stimuli than did sham animals, nor due to a change in motor function, since the maximal startle response of MAM-exposed rats did not differ from that of sham.
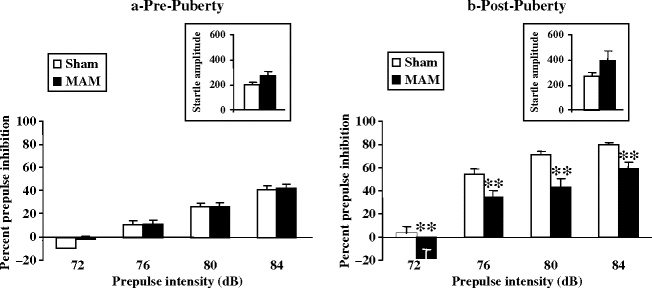

Fig. 2.14.
Effect of prenatal (E17) MAM vs. saline exposure on prepulse inhibition (PPI) of startle reflex. PPI of the acoustic startle reflex (±SEM) was measured at pre- (a) and post-puberty (b). The insets show acoustic startle amplitude. ** p < 0.01 vs. sham group. (Adapted from Le Pen et al., 2006)
Latent Inhibition (LI)
The LI paradigm is based on the phenomenon of reduced conditioning after stimulus pre-exposure. It is used to measure a form of selective attention, namely the ability to ignore irrelevant stimuli.
Procedure
The experiment performed in MAM-exposed rats by Flagstad and colleagues (32) was conducted using an automated shuttle box (42 cm × 16 cm × 20 cm) divided into two compartments by a partition with one opening. On the first day, the animals were left undisturbed in the shuttle box for 50 min with the houselights turned on. On the second day, the animals were divided into groups of pre-exposed (PE) and non-pre-exposed (NPE). The PE rats were subjected to 50 presentations (10–60 s randomized inter-trial interval) of the to-be-conditioned stimulus (cue light on for 10 s). The NPE rats were left undisturbed in the test box with only the houselights on for a corresponding duration. On the third day (test session), the animals were subjected to 100 trials of avoidance learning (10–60 s randomized inter-trial interval). Upon presentation of a light signal, the rat had 10 s to avoid a 0.5-mA scrambled foot shock by moving to the other compartment (avoidance). If the rat did not move to the other compartment within 10 s, a foot shock of a maximum duration of 10 s was delivered. The shock was terminated if the rat moved to the other compartment. The position of the animal and crossings from one compartment to the other were detected by two photocells placed on either side of the dividing wall. The outcome measure was the number of avoidances (i.e., rats moving to the other compartment of the shuttle box after the presentation of the light but before the shock was delivered).
Results
In these experiments (Fig. 2.15), sham animals exhibited a greater number of avoidances in the NPE conditions than in the PE conditions that revealed good latent inhibition processes in those animals. In contrast, MAM-exposed animals exhibited the same number of avoidances in the two conditions, indicating the absence of latent inhibition in those rats (32). The latent inhibition experiment, however, also revealed that under the present experimental setup, MAM-exposed rats had a slower acquisition of the conditioned avoidance.
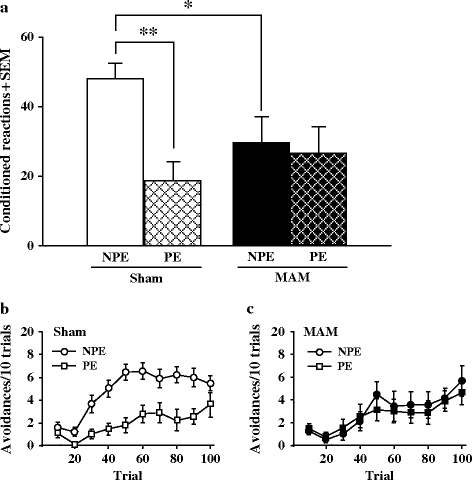

Fig. 2.15.
Latent inhibition (LI) performances in prenatal E17 MAM- vs. saline-exposed rats. (a) Number of avoidance responses (±SEM) during a 100-trial learning session in a two-way avoidance shuttle box in pre-exposed (PE) and non-pre-exposed (NPE) conditions. * p < 0.05, *** p < 0.001 vs. sham group in NPE conditions. The number of avoidances made during the conditioning in 10 trial bins for sham (b) and MAM-exposed (c) animals. (Adapted from Flagstad et al., 2004)
Attentional Set Shifting
In the set-shifting task, rats are required to solve a series of discriminations by attending a particular perceptual dimension of a multidimensional stimulus. A critical discrimination occurs when rats are required to shift to an alternate perceptual dimension after having acquired an attentional set to the previous dimension. The extradimensional shift (EDS) required to solve this discrimination is analogous to what is required to solve the Wisconsin Card Sorting Task, a test that has repeatedly shown impaired prefrontal cortex-mediated executive function in schizophrenia (105). The rodent set-shifting task also requires the prefrontal cortex (106, 107) and, thus, has been used for assessing attentional processes in MAM-exposed rats (30).
Procedure
For this test, Featherstone and colleagues (30) used a black two-compartment chamber (60 cm × 42 cm × 30 cm). Animals had access to either compartment where a small ceramic bowl (7 cm in diameter and 4 cm in depth) was placed. Each bowl was covered with a texture and filled with bedding material that was scented with an odor. Animals were initially trained to reliably dig and retrieve food from two bowls containing a small amount of bedding and a food reward (Fruit loop cereal). Animals were then trained on a simple discrimination (SD), in which food was paired with one stimulus dimension (either odor or texture) but not the other. Training continued until the animals chose correctly over six consecutive trials. The next phase of training involved the full set of discriminations with only one of the two bowls baited. The location of the correct bowl was randomly determined on every trial. The bowls differed according to odor and texture, with food being consistently associated with a particular odor or texture depending on the current discrimination. Discrimination training began with an SD. For this discrimination, only one stimulus dimension was present. In the second discrimination (CD), another stimulus dimension was added, although food remained paired with the original stimulus. The third discrimination involved a reversal of the second discrimination, in which an alternate exemplar of the relevant stimulus dimension was now paired with food. The fourth discrimination required animals to choose between a new series of stimuli, with the previously relevant stimulus dimension still paired with food (intradimensional shift (IDS)). This discrimination was followed by a reversal in which the alternate exemplar of the relevant stimulus dimension was now paired with food. In the sixth discrimination (EDS) the relevant stimulus dimension was changed such that the animal had to learn to attend to the previously irrelevant dimension. The final discrimination involved a reversal of the EDS discrimination in which the animal had to choose the previously irrelevant exemplar of the new stimulus dimension. In a first part, saline- and MAM-exposed animals received initial training (SD, CD, Rev 1, and IDS) with odor as the relevant dimension and were required to make an EDS to texture. In a second part, saline- and MAM-exposed animals received initial training with texture as the relevant dimension, followed by a shift to odor during the EDS. A correct trial was considered to have occurred when the animal successfully retrieved food from the bowl. If the animal dug in the incorrect bowl, the trial was scored as an error. Animals were moved onto the next discrimination once they had made six correct responses in a row. The individual who carried out the set-shifting task was unaware of the group identity of any given animal.
Results
On the attentional set-shifting task (Fig. 2.16), compared to controls, MAM-exposed animals required a greater number of trials to learn the EDS component of the task, indicative of a deficit in shifting attentional set (30). MAM-exposed rats were able to solve an SD and perform an IDS, suggesting that the deficit in EDS ability was not due to a generalized performance or cognitive impairment (30). Since deficits in performing an EDS are found in animals with a compromised prefrontal cortex, as has been shown on the attentional set-shifting task (106, 107), results obtained in MAM-exposed rats are in good accordance with prefrontal morphology and function abnormalities shown in those animals (30, 33, 35, 36, 39).
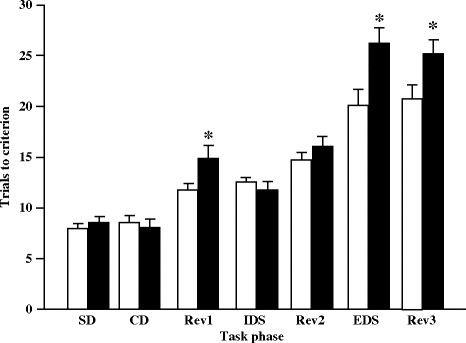

Fig. 2.16.
Mean (±SEM) number of trials to reach criterion on the attentional set-shifting task in prenatal E17 MAM- vs. saline-exposed rats. * p < 0.05 vs. sham. (Adapted from Featherstone et al., 2007)
Nevertheless, it is unclear whether difficulties in shifting attentional set are due to a failure to ignore a previously reinforced dimension or to attend to a previously irrelevant dimension. Lesions of the prefrontal cortex have been shown to selectively increase perseveration to a previously learned dimension in non-human primates (108) and humans (109, 110). Since MAM injection at E17 disrupts prefrontal cortex structure and function, Featherstone and colleagues (30) hypothesized that MAM-exposed animals would likewise show increases in perseveration and that this might serve as the basis for the deficit observed in the present study.
In addition to impairment in EDS learning, MAM-exposed animals were impaired when required to make a simple reversal of a previously acquired discrimination (for two out of three reversals). These results are in good agreement with previous findings. Indeed, difficulties in reversal learning have been reported in E17 MAM-exposed animals within a water maze paradigm (31), as well as in a Y-maze task (36).
Five-Choice Serial Reaction Time (5-CSRT) Task
Visual attentional processes in E17 MAM-exposed rats have been evaluated by Featherstone and colleagues (30) using five-choice serial reaction time task (5-CSRTT). The 5-CSRTT provides the possibility to test the effects of E17 MAM exposure on discrete and somewhat independent measures of behavioral control, including accuracy of discrimination, impulsivity, perseverative responses, and response latencies. Several aspects of the animal performance are assessed (accuracy = % correct), anticipatory responses (premature nose pokes which are thought to be analogous to impulsivity in humans) and perseverative responses (additional nose pokes following a correct response before a new trial is initiated, which are thought to be analogous to compulsive behaviors in humans).
Procedure
In their version of the task, the rats are required to attend and respond to visual stimuli displayed at different positions in an array of five niches placed along the wall of an operant chamber to receive a reward (here, 0.06 ml of a 10% sucrose/water solution) distributed in a magazine/liquid dipper located on the opposite wall. The magazine and light apertures contained photodetectors to detect nose poke entries. First, rats were initially trained to respond to signaled presentations of the dipper. Then, they were trained for 40 daily sessions during which they gradually learn to respond in the appropriate aperture within a certain amount of time. If they failed to respond, or respond in the wrong hole or at an inappropriate time, a short period of darkness (time-out) is presented as a punishment and no reward is delivered (Fig. 2.17).
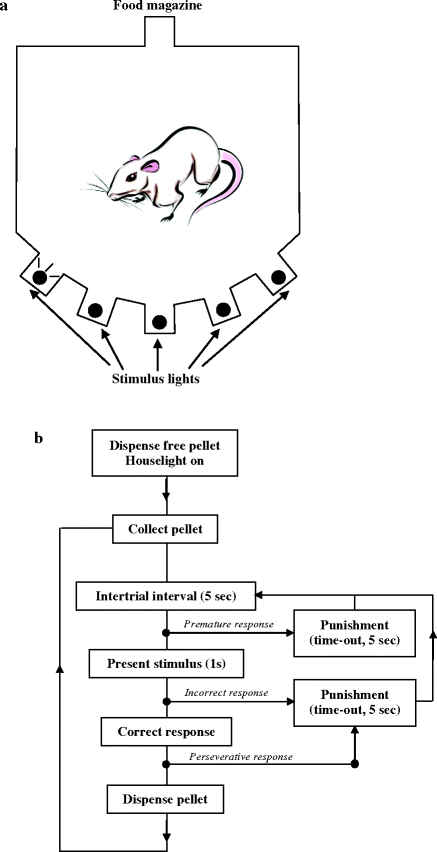

Fig. 2.17.
Schematic illustration of the five-hole box operant chamber. (a) General plan of the 5-choice serial reaction time test apparatus. (b) Flow diagram to illustrate the general task requirements.
Task acquisition: For all subjects, the stimulus duration (SD) was progressively reduced from 30 s until a criterion duration of 1 s was achieved (all other parameters unchanged: ITI 5 s, limited hold 5 s, 100 trials per sessions). At 30 and 20 s, animals were required to perform at least 30 (out of 100) correct responses. At 10 s, the level was increased to 50 correct trials. For 5, 2.5, 1.25, and 1 s, animals were required to make 50 or more correct responses, have greater than 80% accuracy, and less than 20% omitted trials. Animals were trained for a total of 41 sessions in order to reach criterion at the lowest stimulus duration of 1 s.
Behavioral challenges: Once stable performance had been achieved at the 1-s SD, a series of manipulations of the stimulus parameters were employed. First, animals were challenged with different SDs (0.125, 0.25, 0.5, and 1 s). This was performed using a within-subject design, with each test day separated by a maintenance session using 1 s as SD. The purpose of this manipulation was to increase demand on attentional resources. Second, animals were given an additional session using a prolonged ITI (9 s instead of 5 s). This manipulation typically increases premature responding, since animals must delay responding across a longer duration. Finally, animals were given a session of training with variable ITIs (3.5, 5.5, 7.5, and 9.5 s) occurring within the same session.
Results
MAM-exposed rats were able to acquire the task similarly to sham animals since they needed the same amount of time as sham to reach the criterion at each training stage. In addition, at the end of the training, no obvious group differences were observed when comparing sham and MAM-exposed rats in any of the following performances: percent accuracy, percentage of omitted trials, number of perseverative responses, latency (in seconds) to perform the correct response, latency (in seconds) to collect reinforcement, and number of premature responses (30). MAM treatment did not disrupt accurate performance during any of the post-acquisition manipulations (shortened stimulus durations, variable ITI, and lengthened ITI), suggesting that performance in this task is not compromised by MAM treatment. Additionally, the lack of change in omitted trials and response latency suggests that attentional function was spared and, incidentally, indicates that motor and/or motivational functions were unaffected by MAM treatment. Given the effects of MAM treatment on attentional set shifting and reversal learning, as well as on prefrontal morphology, it is surprising that MAM treatment had little effect on the five-choice performance. Indeed, previous work has shown that the prefrontal cortex plays a major role in several aspects of performance in the five-choice task. Hence, prefrontal lesions alter accuracy (111, 112) and can induce changes in perseverative and premature responding (111, 113). MAM-exposed animals did show a trend toward an increase in premature responding during acquisition, suggesting a difficulty in behavioral inhibition (30). However, this change was nowhere near as significant as changes seen in premature responding following other prefrontal manipulations (113). It has to be noticed that rats evaluated in 5-CSRT task by Featherstone and colleagues (30) had been previously evaluated in the attentional set-shifting paradigm. Thus, it cannot be excluded that a facilitation of five-choice performance in MAM-exposed rats could result from their attentional set-shifting experience. Therefore the absence of impact of E17 MAM exposure on sustained visual attention should be confirmed in an independent set of experiment.
2.3.3 Behavioral Flexibility
Impairment in different forms of behavioral flexibility such as set shifting and reversal learning has been frequently observed in schizophrenic patients. Indeed, they display great difficulty in shifting between different rules or strategies on tests such as the Wisconsin Card Sorting task (114–117). These impairments appear to be due in part to an inability to shift attentional set from one stimulus dimension to another, as similar impairments have been observed in patients tested on an intradimensional/extradimensional shifting (IDS/EDS) task (118, 119). Furthermore, a subset of these patients also display impairments in reversal learning, a simpler form of behavioral flexibility entailing shifts between different stimulus-reward associations within a particular dimension (120, 121). In both rats and humans, multiple prefrontal cortex regions and subcortical regions connected to the prefrontal cortex contribute to different component processes of behavioral flexibility.
The orbital and prelimbic/infralimbic regions of the prefrontal cortex mediate some forms of reversal learning in the rat and human (109, 122–126). Then, it is likely that abnormalities in multiple prefrontal regions contribute to the cognitive inflexibility in the E17 MAM model. Thus, behavioral flexibility have been investigated in MAM-exposed rats through a reversal learning procedure that was tested in a Y maze (36) and a water maze procedure that we previously described (31).
Reversal Learning in a Y Maze
Procedure
The test was performed in a Y maze that consisted of a central chamber with guillotine doors opening into three 36-in. runways, each terminating in a food magazine. Adult control rats and MAM-exposed rats were habituated to the Y maze and then trained in E17 the conditional discrimination task. At the beginning of the task, the rat was placed in an arm and the trial initiated by opening the door to the central chamber. Three seconds later, the doors to the choice arms opened and each food magazine was illuminated. A head poke into the CS+ magazine resulted in the termination of the light and delivery of food pellets. A poke into the CS− magazine resulted in the termination of the light and a 15-s time-out. The next trial was initiated from the choice arm of the previous trial; sessions were 30 trials long. After the rat achieved 67% accuracy for 3 consecutive days, the reward contingency was reversed (i.e., the CS+ became the CS− and vice versa). The number of trials to criterion for acquisition and reversal phases was compared between E17 MAM-exposed rats and control rats.
Results
First, relative to sham animals, MAM-exposed rats did not show a deficit in learning the novel discrimination. Indeed, these rats learned the discrimination significantly faster than did control rats, indicating that forebrain circuits involved in basic discriminated approach learning were not affected. However, under reversal conditions, MAM-exposed rats required significantly more trials to reach criterion than did sham animals (Fig. 2.18). Thus, MAM-exposed rats exhibited a significant deficit in reversal learning. Regarding the selective deficit in reversal learning, they observed in E17 MAM-exposed rats, Moore and colleagues (36) suggested that (1) cortical circuits involved in sensory and motor processes and association learning are intact; (2) the faster learning may be indicative of a perseverative response, in which the rats fail to test the non-rewarded arm once the association in the rewarded arm has been experienced; and (3) the perseveration is further revealed when the contingencies are reversed. Their learning phenotype is also consistent with the E17 MAM-exposed rats being more sensitive to the negative consequences associated with the original CS−. Each of these abnormalities implicates frontal cortical function.
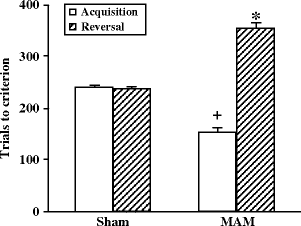

Fig. 2.18.
Reversal learning performances in a Y maze in prenatal E17 MAM- vs. saline-exposed rats. Acquisition of the initial discrimination was significantly faster in MAM-exposed rats. +p < 0.05 vs. sham group in acquisition conditions. However, MAM-exposed rats required significantly more trials to learn the reversal of the discrimination. * p < 0.05 vs. sham group in reversal conditions. (Adapted from Moore et al., 2006)
Reversal Learning in the Morris Water Maze
As previously mentioned, one asset of the water maze tests is that many aspects of performance can be examined, providing several measures of spatial memory. Another asset is its capacity to examine reversal learning as a component of behavioral flexibility processes where rat has to switch from an established strategy to develop a new one.
Procedure
As previously described (see Section 2.3.1), the water maze consisted of a circular, black pool filled with water. The test room contained several permanent extra-maze cues such as the rat housing rack, laboratory table, and posters on the walls. In the experiment performed by Flagstad and colleagues (32), behavioral flexibility can be evaluated between the first and second days of the working memory version of the task. Indeed, the working memory task takes place after the reference memory version of the task (see below) that involves the acquisition of rules about spatial location of the platform that are constant (north quadrant of the pool) across all trials of the task. On the first day of the working memory task, the platform was located in the same position as for the reference memory task but on the second day the platform was positioned in the opposite quadrant. The rationale underlying this task is that animals displaying behavioral flexibility will rapidly learn to search the platform in its new location, while rats impaired in behavioral flexibility will spend more time around the old location. Key measures were path length (m), escape latency (s) to find the hidden platform, percent of path length near (10 cm) the sidewall (percent sidewall), and number of animals not finding the platform within 60 s (non-finders). These measures were recorded using a video tracking system.
Results
On the first day of the working memory task, sham and MAM-exposed animals performed similarly to locate the hidden platform. On the second day, when the platform moved to the opposite quadrant, Flagstad and colleagues (32) showed that MAM-exposed rats performed significantly worse than the controls but only on the basis of the percent sidewall and non-finder parameters (Fig. 2.12). These results suggest that MAM-exposed rats exhibit abnormal behavioral flexibility in a reversal learning version of the Morris water maze task.
2.3.4 Episodic Memory
Human episodic memory refers to the recollection of a unique past experience in terms of its details, its locale, and temporal occurrence (127). Episodic memory deficits among individuals with schizophrenia are well established (128–131). Interestingly, studies suggest that non-human mammals have the ability to build higher-order memory for unique events that incorporate information about what, where, and when, lending strong support to the idea that animals are endowed with episodic-like memory (132–135). Thus, object and spatial recognition tasks as well as spatial reference memory paradigm have been used to evaluate episodic memory in MAM-exposed rats.
Object Recognition Task
Previous studies in schizophrenic patients have demonstrated episodic memory deficits reflected by impaired recognition of visually presented objects (128, 136, 137). In parallel, neuroimaging studies have provided evidence that schizophrenia is associated with abnormal brain activation within the hippocampus, the thalamus, and/or the prefrontal cortex during the recognition of previously seen or new objects (13, 138, 139). Ennaceur and Delacour (135) presented evidence that rodents are able to form an integrated memory for “what,” “where,” and “when” aspects of single experiences by combining different versions of the novelty-preference paradigm, i.e., object recognition memory, the memory for locations in which objects were explored, and the temporal order memory for objects presented at distinct time points. The novel object recognition task (NORT) was used to assess if MAM-exposed animals are able to form an integrated episodic memory for “what.” This test is based on the natural tendency of rodents to explore a novel object by comparison to a familiar one (135). Naive animals will spend more time exploring a novel object rather than a familiar one.
Procedure
The test was performed in an open field (45 cm × 65 cm × 29 cm). On the day before the test, rats were habituated to the test box for 10 min. On the test day, rats were put in the test box, and after 3-min habituation, two similar objects were introduced in the two corners. The time spent investigating the two objects was scored in the next 3-min period (denoted pretest). The rats were then removed and put back in the housing cage. After a delay period of 20 min, the rats were reintroduced to the test box and 3 min later the objects [one similar to that used in the pretest (known) and one the rat had never encountered before (new)] were introduced. The objects were placed in the same position as in the pretest. In the next 3 min, the time spent investigating each of the objects was scored. All scoring was performed using a video tracking system.
Results
The MAM-exposed rats spent significantly less time exploring the objects on the pretest. There was also a main effect of the group in the test session, indicating that the MAM-exposed rats spent less time exploring the objects (Fig. 2.19). There was no main effect of the known/new object factor; however, there was an interaction between the group and the known/new factor. This interaction was caused by a significant difference between the known/new objects in controls, but not in the MAM-exposed rats. Furthermore, there was no difference between the amount of time the animals in each group spent exploring the known object, whereas the controls spent a longer time exploring the new object than the MAM-exposed did. Overall, these results revealed that MAM-exposed rats exhibited object recognition deficits that could be reminiscent of those observed in schizophrenic patients.
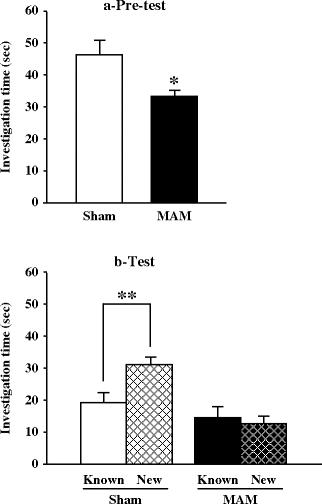

Fig. 2.19.
Object recognition in prenatal E17 MAM- vs. saline-exposed rats. (a) Mean investigation time (±SEM) during the pretest of the object recognition paradigm. * p < 0.05 vs. sham. (b) Mean investigation time (±SEM) of the known and the new object during the object recognition test. ** p < 0.01 known vs. knew. (Adapted from Flagstad et al., 2004)
Spatial Recognition Memory in a Y Maze
Memory for spatial context has been shown to be impaired in schizophrenic patients (140–145). Recently, Da Silva and colleagues (132) proposed that spatial recognition memory in the rodent can be likened to human episodic memory. In rodents, various brain areas, including the cerebral cortex and the hippocampus, are known to be pivotal in spatial recognition memory (146–148). A simple two-trial recognition test in a Y maze was performed to investigate spatial recognition memory in MAM-exposed rats. This test is based on the innate tendency of rodents to explore novel environments (149–151). The paradigm avoids the effects of punishment (such as electric shock) or reward (such as food) that is commonly used in other paradigms and that may have non-specific effects on the results. In addition, it does not require learning of a rule and is independent of locomotor activity; thus, it is useful for studying memory in rodents (150–153).
Procedure
This two-trial memory task is based on exploration of novelty (151). Experiments were carried out in a Y maze which consisted of three identical alleys (40 cm long and 15 cm large with walls 30 cm high), diverging at a 120° angle from the central point. The experiments were performed in a dimly illuminated room. Numerous visual cues were placed on the walls and were kept constant during the experiments. The test consisted of two trials, separated by a 2-h time interval. During the first trial (acquisition phase), one arm of the Y maze was closed, allowing the rats to explore the remaining two arms for 3 min. The position of the closed arm and the introduction arm was randomized. During the second trial (retrieval phase), the rats had access to the three arms for a 3-min period. During this period, for each rat, the time spent in each arm and the total locomotor activity was measured by a video tracking system. The ability of the animals to remember the two arms already visited during the first phase and to explore preferentially the novel arm opened during the second phase. Then, after a 2-h time interval, the control rats are expected to spend more time in the “novel” arm, which remained closed during the first trial, compared with the two “familiar” ones that were already open during the first phase (151).
Results
Before puberty, both MAM-exposed and sham rats showed poor spatial performances revealed by the absence of clear preference for the novel arm (Fig. 2.20). This is consistent with the literature which suggests that rats younger than 40 days exhibit spatial navigation deficits in relation to the immature hippocampus (154, 155). At adulthood, the same MAM-exposed animals spent approximately the same amount of time in the three arms of the maze, in contrast to sham animals that exhibited a clear preference for the novel arm. The data reflect the physiological brain maturation occurring during puberty in control animals. In contrast, in either pre- or post-puberty, the MAM-exposed rats did not exhibit a preference for the novel arm and remained approximately the same amount of time in the three arms of the maze, suggesting that the normal spatial recognition memory skills were not acquired during puberty in the MAM-exposed animals. In addition, MAM-exposed rats spent less time than sham animals in the central zone of the Y maze at both pre-puberty and adulthood, suggesting that memory deficits are unlikely a consequence of anxiety-like behavior. Conversely, when tested in the social interaction test, MAM-exposed rats were not different from controls in the time spent in the central area. So the results in the Y maze could reflect impulsive-like behavior in the MAM-exposed rats. In conclusion, spatial recognition memory, as explored by the Y maze, is thus relevant to follow the late maturation of the hippocampo-prefrontal networks occurring during the rat’s puberty and the related acquisitions of cognitive skills. Y maze results also present interesting similarities with observations of visuo-spatial memory deficits seen in schizophrenic patients (140–145).
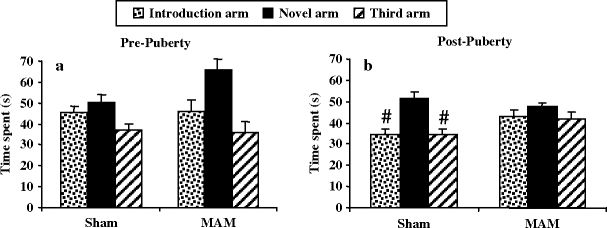

Fig. 2.20.
Spatial recognition memory (Y-maze paradigm) in prenatal E17 MAM- vs. saline-exposed rats. Time spent (±SEM) in the novel arm was compared with the time spent in the introduction and third arms at pre-puberty (a) and post-puberty (b). # p < 0.05 vs. novel arm. (Adapted from Le Pen et al., 2006)
2.3.5 Spatial Reference Memory
Reference memory is regarded as a long-term memory for information that remains constant over repeated trials (156). The retrieval of information, based on spatiotemporal context, from long-term memory is affected in schizophrenia. Morris water maze paradigm is frequently used in rodents to assess reference memory in a spatial context (82, 157). In this paradigm, animals have to navigate to a goal (hidden platform) in an allocentric spatial environment and must build a representation of the goal in relation to distal cues (constellations of cues) to form a cognitive map. Then, they must be able to use this information flexibly regardless of where in the environment the subject begins navigating.
In animals (158) and humans (159), the hippocampus is involved in this kind of spatial learning, because encoding is based on a flexible knowledge of relationships between environmental cues. Hippocampus-dependent spatial memory in animals is thus phylogenetically viewed as a homologue of human episodic memory (160, 161). Thus, in order to evaluate spatial reference memory in MAM-exposed rats, the reference memory version of the Morris water maze has been used by Hazane and colleagues (34) and Flagstad and colleagues (31).
Procedure
The water maze consisted of a circular, black pool filled with water. The test room contained several permanent extra-maze cues such as the rat housing rack, laboratory table, and posters on the walls. In the reference memory task, the rats were trained to locate a hidden platform positioned 1 cm below the surface of the water. The platform remained in a fixed position throughout the test, although the animal’s starting position varied. Key measures were path length (m), escape latency (s) to find the hidden platform, percent of path length near (10 cm) the sidewall (percent sidewall), and number of animals not finding the platform within 60 s (non-finders). These measures were recorded using a video tracking system.
Results
Reference memory capacities measured in MAM-exposed rats revealed various results that could result from differences in either the strain reactivity or the experimental procedure. Thus, in the reference memory paradigm of the water maze task, Flagstad and colleagues (31) showed that MAM-exposed rats performed significantly worse than the controls on days 2 and 3 of testing but only on the basis of the percent sidewall and non-finder parameters. On the last day, however, they performed as well as the controls. Thus, it does not appear that the MAM treatment interferes with spatial working memory, as this should also result in a deficit on the last day of testing. The MAM treatment seems, however, to affect the search strategy of the rats. When the platform is moved, rats spend more time swimming along the sidewall, rather than searching in the central part of the maze where the platform is positioned. On the other hand, in Sprague Dawley rats, Hazane and colleagues (34) observed that MAM-exposed rats were significantly impaired in learning to locate the hidden platform compared to control animals (latency to reach the platform) (Fig. 2.21). Analysis of performance in each group revealed that, in contrast to sham rats, MAM-exposed rats did not improve their latency to locate the platform across trials. This contrasts with previous reports showing no effect of prenatal MAM exposure in Wistar rats using a similar spatial reference memory version of the Morris water maze task and a MAM exposure at E17 (22 mg/kg) (31). This impaired reference memory is of particular interest since patients with schizophrenia were similarly impaired on the hippocampal-dependent hidden-platform version of a virtual Morris water maze task. Indeed, patients were slower and had longer search paths than did controls to find the hidden platform (162, 163).
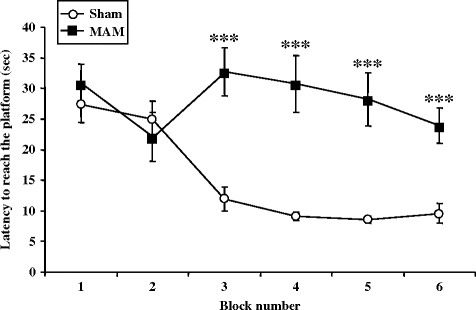

Fig. 2.21.
Reference memory in the Morris water maze in prenatal E17 MAM- vs. saline-exposed rats. Time (sec) to reach the platform in (±SEM) E17 MAM-exposed vs. sham female rats in Morris water maze. *** p < 0.001 vs. sham group. (Adapted from Hazane et al., 2009)
3 Neurochemical Changes Observed in E17 MAM-Exposed Rats
If the behavioral phenotype of E17 MAM-exposed rats is now pretty well characterized, fewer studies have characterized the neurochemical alterations associated with this neurodevelopmental animal model for schizophrenia and its behavioral abnormalities. Structural and/or functional alterations, presumed to reflect abnormal brain development, have consistently been found in schizophrenic patients in several interconnected brain regions such as the prefrontal cortex (164–166), the hippocampus (139, 167–169) and the striatum (170–172). In particular, dysfunctions of glutamatergic and/or dopaminergic neurotransmission have been demonstrated within these structures (164, 169–172) and are thought to play a central role in the pathophysiology of schizophrenia (173, 174). In addition, abnormalities in markers for GABAergic neurons in prefrontal cortex and hippocampus are also well documented in schizophrenia (175–181) with more recent studies suggesting a central role of impairment in the glutamate-GABA interactions, both in the hippocampus and in the prefrontal cortex. In line with these findings, neurochemical abnormalities associated with hypersensitivity to d-amphetamine- and MK-801-induced hyperlocomotion in MAM-exposed rats have been investigated using in vivo microdialysis techniques. In addition, possible abnormalities in GABAergic markers have also been explored in MAM-exposed rats using immunohistochemistry.
3.1 Neurochemical Abnormalities Associated with Hypersensitivity to -Amphetamine-Induced Hyperlocomotion in MAM-EXPOSED Rats
The prefrontal cortex and nucleus accumbens shell regions play a crucial role in mediating the behavioral effects of increased dopaminergic activity in schizophrenia (51, 182–185). In rodents, the nucleus accumbens and prefrontal cortex are strongly implicated in psychostimulant action. Dopamine release in the nucleus accumbens is believed to be the main mediator of the reinforcing and locomotor-activating properties of psychostimulants (186). The prefrontal cortex modulates the nucleus accumbens activity through direct and indirect connections and there is evidence that dopamine release in the prefrontal cortex may be related to the inhibition of reward and locomotor activity (187). Thus, in order to further assess possible corticolimbic dysfunctions associated with d-amphetamine-induced hyperlocomotion in MAM-exposed rats, Flagstad and colleagues (32) investigated changes in dopamine release in the nucleus accumbens and the medial prefrontal cortex after stimulation with d-amphetamine in sham and MAM-exposed Wistar rats.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


