Chapter 25. Geriatrics
Improvements in the control of infectious diseases and in the nutrition of dogs and cats have resulted in a gradual increase in the average lifespan of companion animals. While the maximum lifespan of a given species remains relatively fixed, the average lifespan within a population of domesticated animals can be significantly affected by health care, genetics, and nutrition. By 2002, it was estimated that 30% to 40% of dogs and cats in the United States were 7 years of age or older, and approximately 1⁄3 of these pets were older than 11 years. 1. and 2. The increased number of senior and geriatric pets and the understanding that most of these dogs and cats have been cherished family members for many years necessitates increased attention to the care and nutrition of this portion of our companion animal population. Nutritional goals for aging pets include supporting health and vitality, preventing the onset or slowing the progression of age-related health disorders, and enhancing the pet’s quality of life and, if possible, life expectancy.
AGING VERSUS SENESCENCE
The aging process refers to the natural series of life stages, beginning with conception and continuing through development, adulthood and finally senescence (geriatric stage). Although it is often misconstrued as such, aging is not a pathological process, but rather comprises the normal time-dependent changes that occur during the life of every individual. As many pet owners who cherish their senior dog or cat will attest, living with a healthy older dog or cat can be a wonderful experience. For example, older pets are often well behaved, are enjoyable to live with, and have a well-established set of endearing habits. Other changes associated with older pets are neither positive nor negative, such as the development of a graying muzzle or a slightly reduced activity level. Undesirable age-related changes are those associated with illness, changes in mobility, changes in cognitive function and the development of behavior problems. The deteriorative changes that may negatively affect a senior pet’s overall health and quality of life are referred to as senescence. 3
A variety of theories have been proposed to explain the phenomena of aging and senescence. 4 Several of these focus on genetic controls, such as the somatic mutation and gene regulation theories. Others examine the impact of temporal changes in homeostatic mechanisms of different body systems or the accumulation of the damaging products of cellular aging. For example, the oxidative stress theory postulates that aging is linked to energy expenditure and the cumulative cellular damage caused by the free radicals that are the byproducts of oxidative metabolism. 5. and 6. None of these theories singularly explains all of the changes that are observed during aging; nor are they necessarily mutually exclusive. Most likely, aging and senescence are multifactorial processes influenced by genetics and a myriad of internal and external environmental factors.
LIFESPANS OF DOGS AND CATS
From an evolutionary perspective, domesticated dogs and cats present a unique example of two species that enjoy an extended life stage beyond successful maturation and reproduction. Natural selection works principally on survival and fitness up to and throughout an individual’s reproductive age and this impact diminishes as an animal enters its postreproductive years. In the wild, nondomesticated species typically do not enjoy a long postreproductive life, and older animals rapidly experience increased vulnerability to predation, accidents, and disease. In contrast, companion dogs and cats are protected from the pressures that have historically impacted their progenitor species. Many live well into their geriatric years, a life phase that is accompanied by age-related degenerative diseases and increased incidence of various types of cancer.
Because genes that regulate late-life deterioration have not been subjected to the same pressures of natural selection as those that support reproductive success, it can be argued that seeing an increase in the diseases of senescence is a natural byproduct of domestication. Indeed, several of the theories of aging suggest that the same genes that confer early maturation and a high rate of reproductive success may, at the same time, contribute to illness or disease later in life. 7. and 8. Such genes would persist in a population because of their beneficial impact upon reproductive fitness. In the case of species that invariably die young because of extrinsic (environmental) factors, the negative effects of such genes upon senior fitness would be irrelevant and therefore would be maintained. Conversely, when extrinsic factors are naturally absent or are controlled by human intervention (i.e., the species enjoys a relatively low postreproductive mortality rate), genes that promote longevity may begin to confer a selective advantage. Although the relationship between extrinsic mortality and longevity has been shown to be complex and influenced by other factors, this theory helps to explain the influence of domestication on dog and cat longevity.
The maximum lifespan of the dog is estimated to be about 27 years, but few dogs live to be older than 18 years of age. When all breeds are considered, the average lifespan of the domestic dog is approximately 13 years. 9 Intraspecific variations in longevity are of special interest with dogs because of the wide range in body size and conformation between breeds. Many of the large and giant breeds have a significantly shorter lifespan than the average of 13 years, while small and toy breeds tend to live longer. Numerous studies have shown that when examined across breeds, there is a significant negative relationship between lifespan and mature body size in dogs. 10.11. and 12. The shortened lifespan of giant breeds appears to be a result of the artificial selection for extremely large dogs and accompanying rapid early growth rates. Developmental skeletal diseases related to higher growth rates are an important (but not the only) contributing factor to the shorter lifespan of these dogs. For example, a recent pilot study found that mature body weight was more strongly correlated (negatively) with lifespan in dogs than other measures of size, such as height at the withers. 13 Although this study did not find breed to be a significant factor, they reported that some heavy but short-in-stature breeds, such as the Bulldog, experienced a shorter lifespan due to the influence of inherited structural disorders. The construction of breed standards that enforce a closed studbook and require absolute reproductive isolation has led to inbreeding practices and the overuse of a small number of individuals (usually males) in an already limited gene pool. This has contributed to the propagation of the more than 500 identified inherited diseases of purebred dogs, many of which may contribute to a shortened lifespan for the affected breed. 14. and 15. Although purebred breeding is less widespread in the domestic cat, inbreeding in purebred cats may eventually lead to similar negative effects upon health and longevity. (Recognizing these problems, reputable breeders use careful selection and screening practices to reduce the incidence of genetically influenced health problems in their breed.)
Because breeds of dogs differ in average lifespan and susceptibility to disease, an individual dog’s breed, size, and current health must be considered when determining whether or not he/she should be considered geriatric. General guidelines divide dogs into four categories based upon their adult size, with smaller dogs considered elderly at a later age than larger dogs (Table 25-1). 16 However, the dog’s body condition, activity level, overall health, and the presence of chronic or debilitating disease must always be considered. 17 In general, cats age more slowly than most dogs and do not tend to show breed differences in aging or longevity. The average lifespan of cats that are kept indoors is approximately 14 years, and the cat’s maximum lifespan may be as high as 25 to 35 years. Healthy cats are considered to be geriatric when they are approximately 10 to 12 years old.
| S pecies/S ize | A ge considered geriatric |
|---|---|
| Dogs | |
| Toy/small breeds (5-20 lb) | 11.5 years |
| Medium breeds (21-50 lb) | 10.0 years |
| Large breeds (51-90 lb) | 9.0 years |
| Giant breeds (>90 lb) | 7.5 years |
| Cats | 12.0 years |
When all breeds are considered, the average lifespan of the domestic dog is approximately 13 years. However, many of the large and giant breeds have a significantly shorter lifespan than this average, while some small and toy breeds live much longer. In general, cats age more slowly than most dogs and do not tend to show breed differences in aging or longevity. The average lifespan of cats that are kept indoors is approximately 14 years.
AGE-ASSOCIATED PHYSIOLOGICAL CHANGES
In all animals, the biological effects of aging include a gradual decline in the functional capacity of organs, beginning shortly after the animal has reached maturity. A set of preliminary studies reported age-related changes in laboratory values for the dog and cat (BOX 25-1 and BOX 25-2). 18. and 19. Although the limited number of animals that were examined preclude using these data to make generalized conclusions about age-related changes to blood chemistry and cell data, these data suggest that multiple physiological systems are affected during the normal aging process. However, the clinical significance of these changes is not known and may not be relevant in many cases. In addition, different systems of the body age at different rates, and the degree of compromised function that must occur before clinical signs are seen depends on many factors in a pet’s life. Although one pet may exhibit severe pathological effects of aging by 7 years, another may exhibit no clinical signs even at 12 years. It is also not unusual for more than one chronic disease to be present in a single geriatric pet. This variability necessitates that older animals be assessed as individuals, using functional changes in body systems rather than chronological age to categorize them with the geriatric population.
BOX 25-1
| U nchanged | D ecreased | I ncreased |
|---|---|---|
Alanine aminotransferase Blood urea nitrogen (BUN) BUN:creatinine ratio Cholesterol Creatine kinase Eosinophils Gamma-glutamyl-transferase Lactate dehydrogenase Magnesium Serum chloride Total bilirubin | Albumin Albumin:globulin ratio Creatinine Hematocrit Hemoglobin Lymphocytes Red blood cells Serum calcium | Globulin Platelets Neutrophils Serum potassium Serum sodium Serum triglycerides |
BOX 25-2




| U nchanged | D ecreased | I ncreased |
|---|---|---|
Eosinophils Hematocrit Lymphocytes Neutrophils Serum potassium | Alanine aminotransferase Alkaline phosphatase Aspartate aminotransferase Albumin Albumin:globulin ratio Creatinine kinase Hemoglobin Serum calcium Serum phosphorus White blood cells | Cholesterol Globulin Monocytes |
Metabolic Effects and Changes in Body Composition
An animal’s basal metabolic rate (BMR) naturally slows with aging. This decline is caused primarily by changes in body composition, specifically a loss of lean body tissue. Normal aging in all animals is associated with decreases in lean body tissue (muscle) and total body water and an increase in the proportion of body fat. A decline in body water accompanies the loss of lean body tissue because lean tissue contains 73% water, while adipose tissue contains only 15% water. Age-related changes in body composition may differ for dogs and cats. An early study comparing young and old dogs reported that the body fat content of young dogs was between 15% and 20%, while that of older dogs was between 25% and 30%. 20 In contrast, the body fat content of adult, normal weight cats has been reported to be between 8% and 13%, but did not change significantly between the ages of 1 and 9 years. 21 Conversely, more recent data collected from a colony of young and old cats and dogs found that the body fat of young cats averaged 30% and increased to 35% in cats that were older than 7 years (Table 25-2, Figure 25-1). 22 The higher body fat in the more recent study may reflect the increased incidence of obesity among cats or a difference in feeding regimen and body weight in the different groups of cats studied. In dogs, fat body mass index increased from a mean of 18% in dogs less than 1.5 years to 27% in dogs older than 7 years of age. In both dogs and cats, the percentage of lean body mass declined with age (see Figure 25-1).
| ∗Data collected from 40 young (less than 1.5 years) and old (greater than 7 years) cats, and 36 young (less than 1.5 years) and old (greater than 7 years) Fox Terriers and Labrador Retrievers. (From Hayek MG, Davenport GM: Nutrition and aging in companion animals, J Anti Aging Med 1:117, 1998.) | |||
| L ean mass (%) | F at mass (%) | B one mass (%) | |
|---|---|---|---|
| Young cats | 69 | 30 | 1 |
| Old cats | 64 | 35 | 1 |
| Young dogs | 79 | 18 | 3 |
| Old dogs | 70 | 27 | 3 |
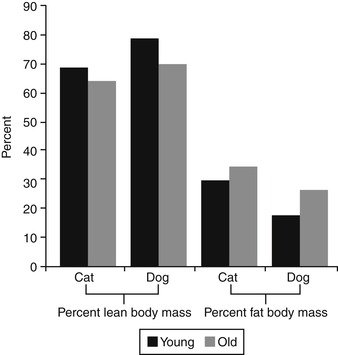 |
| Figure 25-1 (From Hayek MG, Davenport GM: Nutrition and aging in companion animals, J Anti Aging Med 1:117, 1998.) |
Lifestyle factors must be considered when assessing the metabolic rate and energy needs of older pets. While some pets voluntarily reduce their physical activity as they become older, others remain active and athletic well into their senior years. It is estimated that total daily energy requirements may decrease by as much as 30% to 40% during the last third of a pet’s lifespan as a result of both reduced activity and decreased metabolic rate. 23 However, because physical activity helps to offset age-associated losses of lean body tissue, the BMR in older pets that are very active may not decrease significantly. Therefore, while BMR and energy needs generally decrease with aging, once again pets must be assessed individually to determine whether or not a decrease in daily caloric intake is warranted.
Normal aging in dogs and cats is associated with reduced resting metabolic rate, a decline in activity level, decreased lean body mass, and increased body fat. However, lifestyle factors can influence these changes. While some pets voluntarily reduce their physical activity as they become older, others remain active and athletic well into their senior years and maintain a lean and well-muscled body condition. Therefore each dog and cat must be assessed individually to determine the need for a change in daily caloric intake.
Changes in the Integumentary System
The skin loses elasticity and becomes less pliable with age as a result of increased pseudoelastin in the elastic fibers. This loss of elasticity is often accompanied by hyperkeratosis of both the skin and the follicles. Follicles may atrophy, resulting in areas of hair loss. The loss of melanocytes (pigment cells) in the hair follicles and reduced activity of the enzyme tyrosinase results in the production of white hairs, often observed around the muzzle and face of older dogs and cats. Changes in sebum production can result in scaly skin and cause an older animal’s hair coat to become dry and dull. The incidence of skin neoplasia also increases with age. The median age for the development of skin tumors is about 10.5 years in dogs and 12 years in cats. 24
Changes in the Gastrointestinal System
It has been suggested that a number of changes take place in the gastrointestinal tract as animals age. These include reduced salivary and gastric acid secretions and decreases in villi size, rate of cellular turnover, and colonic motility. 23 Although it has been theorized that the aging of the gastrointestinal tract leads to a decreased ability to digest and absorb nutrients, studies on healthy older dogs and cats show conflicting results. A study comparing the digestive efficiency of 1-year-old Beagles to elderly Beagles (10 to 12 years old) found no difference in the older dogs’ ability to digest and absorb nutrients. 25 Similarly, a later study reported no significant difference in digestibility coefficients measured in young adult Beagles and very old Beagles (16 or 17 years old). 26 In contrast, while a more recent study found no significant differences in protein, fat, or energy digestibility between young (less than 6 years old) and old (greater than 8 years old) dogs, there was a trend toward reduced digestibility coefficients in the older group of dogs. 27
Fewer studies have been conducted with geriatric cats. However, data that are available indicate that aging changes in the digestive capabilities of cats may be more significant than those observed in dogs. One study reported no significant differences in overall digestive efficiency between young adult cats and cats that were older than 10 years of age, but the older group of cats had a lower mean fat digestibility coefficient (0.80) than the young group (0.88). 28 This trend has been corroborated by subsequent studies. 27. and 29. When six different age groups of cats were studied, protein digestibility decreased slightly and fat digestibility decreased significantly as cats aged. 27 Together these two trends led to a highly significant linear relationship between age and a decline in diet energy digestibility. The oldest group of cats (12 to 14 years) had significantly lower digestibility coefficients for energy than all of the younger age categories. Interestingly, these older cats (12 to 14 years) were able to maintain normal weight by consuming more food than the younger cats. These results suggest that cats are capable of self-regulating their intake very precisely, even in the face of reduced digestive capacities. Although not determined in this study, possible underlying causes for age-related decreases in digestive capability include reductions in pancreatic enzyme secretion or bile acid secretion in elderly cats. These data indicate that a slight to moderate reduction in gastrointestinal functioning may occur in some healthy older pets. While these changes may not severely affect health, they should be considered when formulating and selecting diets for geriatric dogs and cats.
Changes in the Urinary System
Although a gradual decline in renal function is normal in older animals, a substantial loss of functioning nephrons must occur before changes in renal function are significant. Still, chronic renal failure is a major cause of illness and mortality in geriatric cats and is the third leading cause of death in old dogs. Therefore, age-related changes in renal health have been studied in detail in dogs and cats. 30.31. and 32. One of the first studies evaluated clinical changes in renal function in a colony of Beagles over a period of 13 years. 32 Results showed that nephrosclerosis was the most frequently diagnosed kidney lesion in older dogs. The data from this study also indicated that normal kidney aging may lead to nephron loss of up to 75% before clinical or biochemical signs occur in older dogs. Animals with less than 75% loss are usually clinically normal but may be more susceptible to renal insult than younger animals still possessing renal reserve capacity.
In contrast, another study that compared nutrient utilization and metabolism in young and old Beagles reported no loss of renal functioning associated with aging. 25 These results were supported by a subsequent study that examined the effects of aging and dietary protein intake on renal function and morphology in dogs. 33 All of the dogs were between 7 and 8 years old at the start of the study and were uninephrectomized to reduce renal mass by 50%. This procedure was included in the study design because a reduction of renal mass makes residual renal tissue more vulnerable to insult and would be expected to exacerbate any effects of aging or diet. Over a 4-year period, none of the dogs showed a decline in renal functioning. Age-related changes to the kidneys included the development of moderate renal lesions, but these were not affected by diet and did not significantly affect renal functioning. When the dogs in this study were compared with young dogs that had been uninephrectomized, the older dogs had compensatory responses to uninephrectomy that were indistinguishable from those of the young dogs. However, the older dogs did show a blunted renal response to a protein meal when compared with younger dogs. Results of these studies illustrate the importance of evaluating geriatric patients as individuals when assessing renal function. Aging alone is not associated with clinical signs of reduced renal functioning or chronic renal disease.
When renal disease does occur in older pets, it directly affects nutrition and dietary management because clinical kidney insufficiency is associated with weight loss, muscle wasting, altered plasma protein profiles, decreased caloric and nutrient intake, intestinal malabsorption, and reduced assimilation and use of nutrients. The accumulation of the metabolic end products of protein, of which urea is the most abundant, may further contribute to the development of the clinical and physiological abnormalities of renal failure. Dietary modification can be instituted to minimize the accumulation of these end products in the bloodstream and to slow progression of disease, while still supplying adequate energy and protein to maintain weight and minimize muscle wasting (see Section 5, pp. 417-425 for a complete discussion).
Changes in the Musculoskeletal System
As discussed previously, aging is associated with a decline in the percentage of lean body (muscle) mass. Bone mass also decreases slightly as animals age. Both the number and size of muscle cells decrease with age, and the cortices of the long bones become thinner, dense, and brittle. The composition of the articular cartilage matrix changes with age; specifically reduced number of chondrocytes leads to decreased production of glycosaminoglycans, type 1 collagen, and chondroitin sulfate. Aging cartilage becomes less resilient and has limited ability to regenerate in response to intense activity or trauma. In some cases, the cumulative and pathological outcome of these changes is the development of osteoarthritis. Following an initial peak in incidence due to developmental bone diseases in young dogs, the risk of developing joint problems increases in dogs that are 5 to 7 years of age or older. 34 The presence of joint pain associated with osteoarthritis may also affect a pet’s appetite and ability to eat. Along with medical therapy, there are several nutraceuticals that may aid in the management of arthritis in older pets (see Chapter 37, pp. 502-506 for a complete discussion).
Changes in the Immune System
Similar to humans and other animals, immunocompetence declines with age in dogs and cats. 35.36. and 37. Cell-mediated immunity (i.e., T-cell immunity) is the most severely affected component of the immune system. Reduced T-cell responsiveness is implicated as having a role in numerous degenerative diseases such as osteoarthritis, cancer, and increased susceptibility to infection. Studies with dogs have also demonstrated age-related declines in mitogen stimulation, chemotaxis, and phagocytosis. 36 Older dogs may have decreased white blood cell and immature neutrophil counts and increased mature neutrophils and circulating levels of immunoglobulin G. 38 Although only two breeds were compared, there is evidence that the rate of decline in the immune system may differ between breeds of dogs. 39 Older cats may exhibit reduced mitogen response, and delayed type 1 hypersensitivity and antibody responses. 40 Because the free radical theory of aging predicts that the cumulative effects of free radical reactions and their byproducts lead to cellular damage and death, the potential exists to ameliorate age-related dysregulation of the immune system through nutritional intervention (see pp. 270-271).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


