CHAPTER 21 Flavivirus Infections
Before the North American epizootic of West Nile virus (WNV), several features of flavivirus outbreaks were noted by scientists in the public health community.1 First, outbreaks with these viruses were occurring in new locales over the previous 20 years. Second, outbreaks with apparent clinical disease were becoming more frequent. Third, enhanced pathogenicity for humans was often observed. Fourth, development of better molecular technologies demonstrated increased viral variation within this group of viruses (likely accounting for the trends just mentioned).
Emergence of new diseases or new outbreaks of previously described diseases are largely the product of globalization. This has created an interface of exotic disease and new interfacing with people, pests, and animals traveling over unprecedented distances.1 Before 1999, the U.S. equine practitioner had little familiarity with flaviviruses. Horse owners typically did not even know these diseases existed. After WNV was first identified in the United States, the widespread outbreak in humans and horses residing in middle and northern latitudes of the North American continent was not predicted.2–4
Some of the most important scientific landmarks in human and veterinary health have centered on flaviviral infections. In 1900, Walter Reed and colleagues demonstrated that the causative agent of yellow fever (YF) was a filterable agent or virus. This was also the first agent that was determined to be transmitted by arthropods,5 allowing the eradication of YF from coastal cities of North America and Cuba. The molecular sequencing of uncultivable hepatitis C virus (HCV) allowed development of screening tests, which has resulted in a significant decrease in transfusion-related transmission of HCV.6,7
ETIOLOGY
The family Flaviviridae consists of a pathologically active group of viruses composed of three genera that are found worldwide.8 This group of viruses has the distinction of containing some of the most important human pathogens in the world. The genus Flavivirus contains many viruses (approximately 70), which are usually transmitted by ticks or mosquitoes (some are through direct contact, or the vector is unknown) and organized into groups according to cross-neutralization with polyclonal hyperimmune mouse ascites (Table 21-1). About one fourth of these viruses are of veterinary importance. The other two genera have viruses of veterinary and human importance, with the genus Pestivirus containing the ubiquitous bovine diarrhea virus (BVD), and Hepacivirus containing the human pathogen HCV. At least half the members of Flaviviridae are zoonotic.
Table 21-1 Partial Taxonomic Structure, Host, and Primary Vector of Genus Flavivirus, Family Flaviviridae*
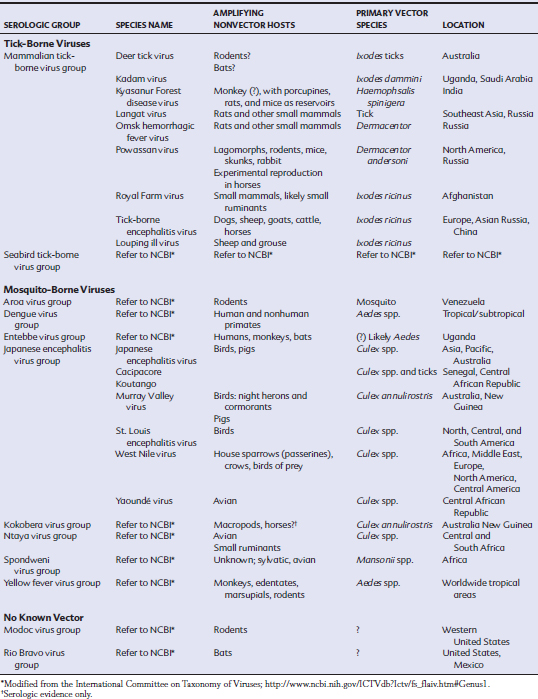
The members of the Japanese encephalitis (JE) serogroup that are most likely to cause overt disease in horses are JE virus (JEV), WNV, and Kunjin virus (KV; an Australian flavivirus, now considered a variant of WNV).9 Disease in horses caused by Murray Valley fever (MVF) is geographically restricted to the South Pacific and is sporadic in occurrence.10–12 Several other members of this group and those belonging to the other major groups of flaviviruses have been detected serologically in horses, with limited reports of clinical disease. Published reports of experimental reproduction of disease in horses are lacking for many of these viruses (see Table 21-1). The following discussion emphasizes WNV and JEV.
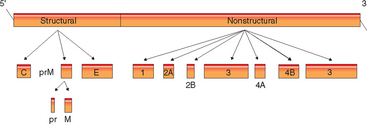
As with other members of the Flaviviridae, WNV (and JEV) are positive–sense, single-stranded ribonucleic acid (RNA) viruses measuring approximately 50 nm.6 The virions are spherical and enveloped with the C protein, making up a nucleocapsid of about 25 nm. Electron microscopy reveals an icosahedral symmetry of the envelope and capsid of these viruses. An approximately 11-kilobase (kb) genome contains a single open reading frame (ORF) that is translated in its entirety and cleaved into 10 viral proteins by both cell and viral proteases13,14 (Fig. 21-1). There are three structural and seven nonstructural proteins; the structural proteins include the capsid (C), premembrane (prM) and membrane (M), and envelope (E) proteins. The nonstructural (NS) proteins, numbered 1 through 5, are cleaved after translation to form NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 and are required for viral replication and assembly.
The final M protein and the E protein are important in virulence for the JE group15–20 (see Fig. 21-2). The M protein is formed from a precursor protein (prM protein), which is modified as immature virions are secreted through the Golgi network of the cell, leaving the C-terminal portion of the protein inserted in the envelope of the mature virion. The E protein is only secreted in its native conformation through association with the prM protein. The E protein is the immunodominant viral protein and exists in the virion as a β-pleated sheet arranged head to tail, with the distal ends anchored in the membrane. This protein is dimeric, held together with intermolecular disulfide bonds, and lies flat against the lipid bilayer. This large protein is important in receptor ligand binding and fusion to cells, the latter being pH dependent. There are three major domains of this protein in WNV. Domain II contains a region important to virus binding in the brain. Domain III is important for vector and host virulence.21 In WNV, glycolysis of the E protein is strain dependent and is associated with virulence. viral binding to glycosaminoglycan on cells changes virulence in both JEV and WNV.
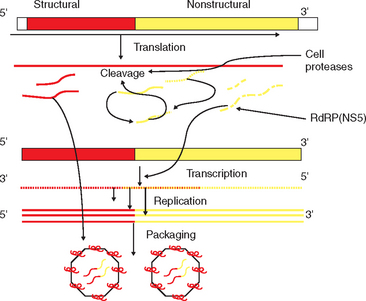
The NS proteins in flaviviruses are structurally and functionally similar and are involved in synthesis of viral RNA.22,23 The glycoprotein NS1 is essential for virus function and appears to be important for cell activation as part of viral synthesis.24–26 NS1 is found on cell membranes of infected cells and must interact with NS4A in this process. NS2A is formed by cleavage of full-length NS2. Changes in the C-terminal of this protein results in loss of viral replicative ability.27 In addition, NS2B complexes with NS3 to form a serine protease.27–29 The NS3 protein is highly conserved between flaviviruses and, at the N-terminal, encodes a serine protease with sequences consistent with the trypsin superfamily. The C-terminal of this protein has sequences typical of RNA helicases and triphosphatases.30 The NS4b protein appears to block antiviral cytokines.31 The NS5 protein is essential for viral replication by forming the “cap” at the 5′ end of a genome. Viruses, as opposed to eukaryotic cells, have a type I cap at the end of the genome, which in a cytoplasmic virus such as WNV, must be formed solely by viral proteins. In addition, this is the site of the viral RNA-dependent RNA polymerase (RdRp), an essential protein for formation of negative-strand RNA from the genome of the positive-strand “parent” RNA virus.31,32
West Nile virus (and members of the JE serogroup) are thought to infect the cell through glycoprotein receptors that are likely highly conserved by hosts.32–34 After receptor-mediated endocytosis, there is fusion of the viral membrane with membranes of the endosomal vesicle, and the nucleoprotein is released into the cytoplasm. After translation, the serine protease NS2B-NS3A, along with cell proteases, cleaves the polyprotein at multiple sites to generate viral proteins (Fig. 21-2). The RdRp copies the negative-strand RNA from the genomic RNA template. These negative-strand RNAs become templates for the synthesis of new genomic RNAs. There are likely alternating periods of replication and translation until a sufficient pool of structural proteins has accumulated. Once there is a pool of genomic RNA, virion assembly occurs in the rough endoplasmic reticulum (ER) membranes. Immature virions, still with the prM, accumulate in vesicles and are transported through the host secretory pathway, where the E and prM proteins are modified. Virions are transported to the plasma membrane in vesicles and released by exocytosis. Mammalian cells will release progeny virus within 10 to 12 hours after infection.
EPIDEMIOLOGY
Life cycle
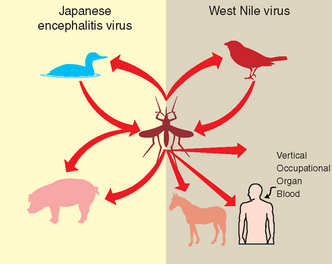
Japanese encephalitis serogroup viruses are vector-borne diseases, with transmission occurring to avian and mammalian hosts from blood meal–seeking mosquitoes35 (Fig. 21-3). Virus is either maintained or cycled between vectors, and biologic amplification occurs within the vector species. Vertical transmission within vectors must occur for maintenance of the respective virus within a geographic area.36 The primary nonarthropod reservoir hosts for these viruses, in which the virus is amplified and transmitted to vectors, are birds. Horses and humans are “dead-end” hosts and do not amplify the virus in quantities sufficient to infect mosquitoes. In JE, swine are considered important amplifying hosts.
Additional modes of transmission have been identified in the recent North American WNV outbreak. First, transmission through oral ingestion has been proven in both avian and mammalian hosts, and oral and cloacal shedding has been demonstrated in birds.37–41 Second, WNV may be transmitted through contaminated blood transfusion or organ transplantation if donors are viremic.42–46 Third, vertical transmission through placenta and milk has been demonstrated in people.44,47,48 This last feature is important in JE and several JE serogroup virus infections.
Recent Epizootology
The largest documented outbreak of equine neurologic disease caused by a flavivirus began in 1999 with WNV encroachment into the United States (U.S.). West Nile virus was first detected in 1999 in New York City. Since that time, more than 25,000 cases of equine West Nile encephalomyelitis (WNE), with an estimated 30% to 40% case-fatality rate, have been reported in horses in the U.S. Overt clinical disease is still common in most states, with 3000 human cases and 914 equine cases reported during the 2005 arbovirus season.49 In 2005, 119 human deaths occurred as a result of WNV infection.50 California has reported equine mortality rates of greater than 40% for 2 years.
During 1999, WNV caused human disease in the U.S. during the weeks of July 25 to September 12, and initial surveillance identified bird mortality and equine infection as features of the disease.51,52 Illness was detected in 60 people, with seven deaths occurring in four New York boroughs and two neighboring New York counties. During that first year, WNV was detected in four states and 12 counties by human and equine serology and testing of live and dead birds. Evidence of encroachment based on human, equine, avian, and mosquito testing was detected in 12 states and 133 individual counties in 2000, with slightly more equine cases (65) and fewer human cases (21) reported in three of those states.53,54 For that year, 4139 dead birds representing more than 76 species were reported as WNV infected. Other seropositive mammals that year included bats, rodents, rabbits, cats, raccoons, and skunks.
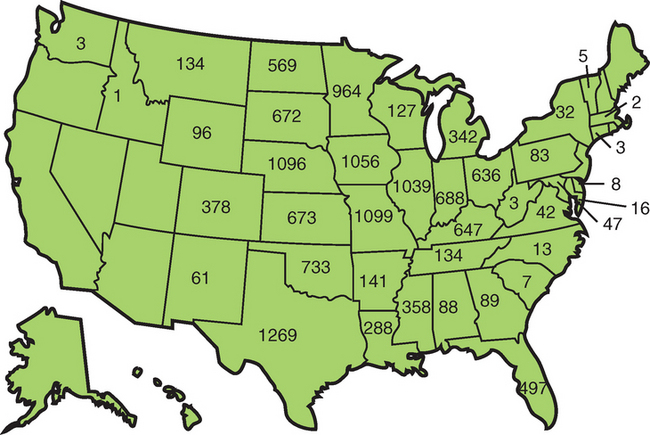
The WNV outbreak reached epizootic proportions in 2001 when many equine cases and high levels of bird mortality were reported in the southeastern U.S., especially in southern Georgia and Florida.55 WNV was reported in 27 states and 359 counties, with an expansion of the arbovirus season from June 27 to December 18 in 2001. Human illness was confirmed in 66 people, and 7333 dead birds tested positive for WNV. Corvid susceptibility became more apparent, with 5154 positive crows and 966 positive blue jays. In 2001, there were 733 confirmed cases of equine encephalomyelitis in 19 states and 127 counties.55 In 2002, explosive WNV activity occurred in 2289 U.S. counties and 44 states, with 3389 cases of WNV disease reported in people, of which 2354 persons had neuroinvasive symptoms resulting in 201 deaths.56 There were 4717 horses reported with confirmed WNV disease, with 30% to 35% mortality in affected horses57 (Fig. 21-4).
By 2005, WNV had been identified in all the 48 continental U.S. states.58 Canadian provinces reporting disease included Quebec, Ontario, Manitoba, Saskatchewan, and Alberta, with New Brunswick and Nova Scotia reporting evidence of WNV-positive birds.59 Serologic evidence of WNV has been reported in the Latin American countries of the Dominican Republic, Mexico, Guadeloupe, El Salvatore, Puerto Rico, Cayman Islands, Jamaica, Belize, and Cuba.41,60–65 The incidence of equine and human disease appears low for Central and South America and the Caribbean compared with the United States.66
Japanese encephalitis virus causes 30,000 to 50,000 human encephalitis cases annually worldwide, with endemic areas including China, the southeast region of the Russian Federation, South and Southeast Asia, and Australia. Exact numbers of horses with clinical JE are difficult to ascertain; however, there are reports of JE isolation from horses in Taiwan, China, Pakistan, and Australia in the literature since the 1980s. Outbreaks in horses have also been reported in India, Nepal, the Philippines, Sri Lanka, and Northern Thailand. Seroconversion of young horses over their first year of exposure in Hong Kong is as high as 63% in some locales.
The spread and yearly incidence of JE serogroup viruses coincides with the availability of vectors and reservoir hosts with transmission potential. Thus, outbreaks are seasonal and reflect mosquito activity. Culex species of mosquitoes are considered the primary mosquito vector for the JE serogroup.41,67–70 WNV has been detected in approximately 60 species of North American mosquito, but the overall vector efficiency (moderate to high) and wide range of feeding activity of the Culex indicate that the North American WNV outbreak is propelled mainly by this genus.71 Most of the data supporting this conclusion is based on vector efficiency studies under laboratory conditions, experimental feeding studies, and frequency of identification of WNV in mosquito pools.71,72 In the northeastern U.S., more than half the WNV-positive mosquito pools are Cx. pipiens.73–77 In the western U.S., populations of the highly efficient Cx. tarsalis constitute the majority of positive pools, with Cx. pipiens the next most frequently found Culex species.78–80 In the southeastern U.S., Cx. quinquefasciatus and Cx. nigripalpus have the highest WNV infection rates.81–85 In the southwestern U.S., epidemics are most often associated with positive mosquito pools of Cx. quinquefasciatus, Cx. tarsalis, and Cx. pipiens.77,86–89 Culex restuans, frequently identified as part of the “Cx. pipiens” complex, is often one of the top five positive species.71,89
Although Culex is important in the epidemiology and spread of WNV, relatively little is known regarding the actual vector of transmission to the horse. Blood meal analysis suggests that Cx. pipiens mosquitoes are primarily avian feeders. Mammalian feeders include mainly Anopheles quadrimaculatus, Coquillettidia perturbans, and Aedes albopictus.90,91 Analysis of Cx. quinquefasciatus has detected both human and bird blood meals, whereas Ae. albopictus most often contains human blood.92 Culex salinarius reportedly has the most variable feeding habits, which is important for transmission between different host species. Culex pipiens and Cx. salinarius are likely the most important bridge vectors.
Experimental infection of horses through mosquito transmission studies is accomplished with Ae. albopictus, a common mammalian feeder and moderately efficient vector of WNV.93,94 In studies thus far involving low numbers of horses, 9 of 10 horses became viremic, and all seroconverted to the virus. The significance of this vector under natural conditions is unknown. Members of the Culex tritaeniorhynchus group of mosquitoes are the most important vectors for JE, and this mosquito was used in the experimental transmission and reproduction of disease in horses.
Several species of ticks have been investigated for potential to transmit WNV. Transtadial transmission was demonstrated in one study of Ixodes ticks, but failed to occur in a second study with these ticks.95,96 Carios capensis can transmit WNV under experimental conditions to ducklings, and Ornithodoros moubata can transmit WNV under experimental conditions to mice.96,97
A reservoir host is one in which a pathogen is amplified in vivo so that it can be transmitted to a vector species.75 A blood meal taken from a mammal containing 105 to 107 plaque-forming units per milliliter (PFU/mL) of WNV results in infection of 30% to 100% of feeding mosquitoes, respectively. Humans voluntarily infected (with the Egyptian strain of WNV) developed virus titers of 103 to 105 PFU/mL. In horses the maximal titer after infection with this strain was similar.98 Titers greater than 104 PFU/ml WNV in plasma are inconsistently detected in cats, and this species may be capable of short-term infectivity for mosquitoes.99
To date, more than 300 species of birds have been reported as WNV positive in the U.S., with 16 new species identified during the 2005 season.50,100 High levels of viral amplification occur in many bird species, especially Passeriformes (e.g., songbirds) and Charadriiformes (e.g., shorebirds);40 the house sparrow is considered the most important amplifying host for WNV (Table 21-2). Although the crow is one of the most competent vectors, sparrows have a lower mortality and thus longer days of infectious virus. Strigiformes (e.g., owls) and Falconiformes (e.g., falcons) develop a viremia of shorter duration, but sufficient to infect mosquitoes. Psittaciformes (e.g., parrots) and Galliformes (e.g., game birds) develop the lowest viremias. By contrast, Anseriformes (e.g., waterfowl) are considered the most efficient avian reservoirs of JE and are essential for the spread of this virus through avian flyways.
Table 21-2 Top U.S. Avian Hosts for West Nile Virus by Vector Competence
| ORDER (FAMILY) | COMMON NAME (TOP 8 SPECIES BY LEVEL OF VIREMIA) | MORTALITY |
|---|---|---|
| Passeriformes (Corvidae) | Blue jay | High |
| Passeriformes (Icteridae) | Common grackle | High |
| Passeriformes (Fringillidae) | House finch | Moderately high |
| Passeriformes (Corvidae) | American crow | High |
| Passeriformes (Passeridae) | House sparrow | Low to moderate |
| Charadriiformes (Laridae) | Ring-billed gull | Moderate |
| Passeriformes (Corvidae) | Black-billed magpie | Moderate |
| Passeriformes (Corvidae) | Fish crow | Low to moderate |
Modified from Komar N: Adv Virus Res 61:185-234, 2003.
Although corvid susceptibility has been described as unique to the North American outbreak, early studies with the Egyptian WNV strain produced high mortality in crows.101,102 Corvids develop very high viremia with very high mortality rates from WNV, but even with their high mortality, they are likely efficient reservoirs.37,39,40,103 Corvid susceptibility to WNV in the U.S. outbreak is an important indication of local activity and encroachment in the U.S. Dead bird counts are an important surveillance tool.
Occurrence of disease caused by JEV and WNV in horses and people reflects vector activity, seasonal in temperate regions and year-round in subtropical and tropical regions. Intense virus activity in the U.S. begins in July, with a peak incidence in September and October.104–107 Temperature-dependent spatial modeling supports these disease dynamics, with risk increasing from 25% in late August to greater than 75% by the second week of September.108–110 A drop in ambient temperature with soft frost usually results in a rapid decrease in reporting activity.111,112 The appearance of disease from JE is actually highly variable depending on the locale. Seasonal occurrence of disease in specific locales should be considered to facilitate timing of equine athletic events and to tailor vaccination regimens appropriately.
Older people appear more susceptible to neuroinvasive disease from both JEV and WNV. This age bias in reporting appears true, at least for WNV, in horses.41,50,53,113–115 Although men are more frequently affected with neuroinvasive disease, no breed or gender predilection seems to exist in horses. In one study of horses with WNV encephalomyelitis, female horses were 2.9 times more likely to die than male horses with neurologic signs.116–118
The remarkably explosive North American outbreak of WNV has introduced new potential hosts for the virus. Seropositive, free-ranging mammals include the big brown bat, little brown bat, eastern chipmunk, eastern gray squirrel, eastern striped skunk, white-tailed deer, and the brown bear.41,75,100,119 Neurologic disease has been confirmed as WNV in gray squirrels and fox squirrels.120 Alligators can have an extremely high titer of viremia and may be an important reservoir for WNV in the southeastern U.S.119 There are reports of both farmed and free-ranging alligators with neurologic signs from which WNV has been isolated. In farm-raised alligators, cloacal shedding of virus has been demonstrated, with oral infection likely.
Serologic evidence of natural infection has been demonstrated in domestic dogs and cats.37,38,121 Experimental infection of cats resulted in a mild transient fever in some cats and a short-term viremia high enough to possibly transmit to mosquitoes.121 Oral transmission to cats has also been documented. New world camelids develop neurologic disease with natural exposure to WNV.122,123
PATHOGENESIS
Mammalian disease caused by infection with the JE serogroup viruses uniformly demonstrates predilection of these viruses for nervous tissues. Neurologic disease in the horse consists of changes in mentation, signs consistent with spinal cord abnormalities, and defects in cranial nerves of the hindbrain.124–134 The change in behavior likely results from viral infection and pathology induced in the neurons of the thalamus, medulla, and pons, with limited viral load in the cerebrum.126,127,135 Although the thalamus integrates all sensory input to higher centers, lesions within the midbrain and rostral pons may affect the reticular formation, which has an important role in regulation of consciousness.134,136 The reticular formation projects to the thalamus, which in turn sends diffuse projections to the entire cortex.134 This formation also travels directly to the base of the forebrain, which is the source of cholinergic stimulation to the entire cerebral cortex. Disturbances of the reticular formation and the midbrain may induce behavioral changes ranging from severe aggression to somnolence and even coma.
West Nile virus–induced motor deficits are multifocal, asymmetric, and primarily characterized by weakness and ataxia.126–128,132,135,137,138
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree