CHAPTER 13 FERRETS
The ferret, Mustela putorius furo, has been domesticated for more than 2000 years. Its origin and early use are unclear, but historically it has been utilized for rabbit hunting and rodent control, particularly in the British Isles, Australia, and New Zealand. Ferrets have proven to be an important model in biomedical research, and much of what is known today about the ferret is based on this use. In recent years, its popularity as a household pet has risen dramatically because of its amicable nature, small size, and relative ease of housing and care. As the number of pet ferrets increase, so does the need for proper veterinary care. This chapter is written to familiarize the veterinarian with basic knowledge of ferret biology and care, medicine, and surgery.
BIOLOGY
The domestic ferret belongs to the order Carnivora and the family Mustelidae.1 Other members of this family include the weasel, stoat, otter, mink, and skunk. The ferret is most closely related to, and may be a descendent of, the European polecat, M. putorius, or the Steppe polecat, M. eversmanni. It should not be confused with the North American black-footed ferret, M. nigripes. The name Mustela putorius furo was derived from the Latin words putor and furonem, meaning “stinky thief,” and accurately describes its mischievous behavior and musky odor.
There are several color variations that can be found in the pet trade. The most common ferret color is the sable, characterized by dark guard hairs, mask, legs, and tail, and a cream-colored undercoat. Also quite common are the albino; the black-eyed white, with a white coat and dark irises; and the cinnamon, with beige guard hair, no mask, and a cream-colored undercoat. Silver ferrets, when young, have dark gray guard hairs, an indistinct mask, and some white present on the ventrum. As they age, the coat progressively turns to a black-eyed white variation. Varieties that have white paws are considered “mitts,” and those with white heads and bibs are called “pandas.” It has been reported that breeding silver mitts together or with black-eyed whites can produce offspring with congenital abnormalities.1 A healthy ferret coat is shed twice a year, resulting in a long, thick winter coat and a shorter summer coat.
Ferrets measure 44 to 46 cm from nose to tail tip.2 Males, also known as hobs, are larger than females, or jills, with the average male weight of 1 to 2 kg and the average female weight of 0.5 to 1 kg. The weight may be less in the neutered male or greater in the spayed female. There is a seasonal fluctuation in body weight of 40% loss in the summer and gain in the winter in intact animals.3 Refer to Box 13-1 for additional physiologic data.
BOX 13-1 Normal Physiologic Values of the Ferret
Data collected from SA: Ferrets: Basic anatomy, physiology, and husbandry. In Hillyer EV, Quesenberry KE, editors: Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery, Philadelphia, 1997, WB Saunders; and Fox JG: Normal clinical and biologic parameters. In Fox JG, editor: Biology and Diseases of the Ferret, ed 2, Philadelphia, 1998, Lippincott Williams and Wilkins.
| Life span | 5-8 yr3 |
| Body temperature | 100°–104° F3 |
| Heart rate | 180-250 beats/min3 |
| Urine volume | 26-28 ml/24 hr18 |
| Respiratory rate | 33-36 /min18 |
| Age of sexual maturity | 6-12 months18 |
| Gestation | 42 + 2 days18 |
| Litter size | 1-18 (average 8)18 |
| Birth weight | 6-12 g18 |
| Eyes open | 34 days18 |
| Weaning | 6-8 weeks18 |
The musky odor of ferrets originates from the presence of a large number of sebaceous glands in the skin.3,4 The number of these glands increases in the breeding season in the intact animal, resulting in a stronger body odor, yellow discoloration, and oiliness of the fur. The anal glands also produce a strong, pungent odor but are rarely expressed except when frightened or traumatized. The anal glands are usually removed at an early age in the United States at the breeding facility. Ferrets are unable to sweat because of a lack of sweat glands in the skin.3,4 Overheated ferrets often flatten their bodies on a cool surface to dissipate body heat. Despite their musky odor, ferrets should be bathed no more than once or twice a month; otherwise, excessive drying of the skin and subsequent pruritus could result.
The head of the ferret is compressed dorsoventrally, elongated rostrocaudally, and lacks sutures in adults.2 Like the dog, the zygomatic bones of the ocular orbits are open.1,2 Ferrets have powerful jaws, large canines, and reduced molars. The mandibular teeth sit within the maxillary teeth when the jaws are closed, which allows for a shearing action when eating.2 The dental formula of the ferret is: I-3/3, C-1/1, P-4/3, M-1/2. Box 13-2 gives the ages at which the permanent teeth erupt.
BOX 13-2 Age of Tooth Eruption
Data collected from Evans HE, An NQ: Anatomy of the ferret. In Fox JG, editor: Biology and Diseases of the Ferret, ed 2, Philadelphia, 1998, Lippincott Williams and Wilkins.
| Days | Teeth |
|---|---|
| 20-28 | Deciduous |
| 50-74 | Permanent |
| 50 | Upper and lower canines and 1st lower molars |
| 53 | 1st upper molars |
| 60 | 2nd, 3rd, 4th upper premolars and 2nd lower premolars |
| 67 | 3rd lower premolars |
| 74 | 4th lower premolar and 2nd lower molars |
The spine of the ferret is long and flexible, with large vertebrae: numbers C7, T15, L5 (6 or7), S3, and Cd18.2 There are 15 (sometimes 14) paired ribs, with 10 pairs attaching to the sternum and the last 5 pairs attaching to each other. The costochondral junction is usually swollen from rapid growth and can be palpated. The clavicle is reduced in the ferret. Each of the ferret’s feet has five clawed digits. Unlike the cat, the claws are not retractable and require periodic clipping. In the hob, the glans of the penis contains a J-shaped os penis, which is attached at its base to the corpus cavernosum. The function of the corpus cavernosum is to stiffen the penis during intromission and possibly dilate the cervix.
The ferret heart is cone-shaped and lies between ribs 6 and 10. From a ventrodorsal view, the apex of the heart lies to the left of midline.2 The ligament connecting the heart to the sternum is often heavily laden with fat, giving the heart a raised radiographic appearance from the lateral view. The ferret is also unique in having a single innominate or brachiocephalic artery arising from the arch of the aorta, rather than two carotid arteries.5 Before the brachiocephalic artery’s exit from the thoracic cavity, it branches into right and left carotid arteries that continue cranially along each side of the neck to supply the structures of the head. There are differing views as to whether or not this anatomic adaptation allows for continued blood flow to the head even when a ferret is turned 180 degrees.3,5
The respiratory tract of the ferret is also unique in several ways. The trachea, bifurcating at the fifth intercostal space, is quite long and large in diameter, characteristics that decrease the central airway and pulmonary resistance.1 Ferrets also have a large total lung capacity and respiratory reserve compared with other animals of similar size. Even more interesting is that the airways grow in length and diameter with an increase in total body length.1,2 This characteristic and the similarity of the tracheobronchial wall to that of humans makes it a good comparative model for respiratory research.1,5
The ferret’s gastrointestinal tract differs from the dog and cat in the absence of a cecum, and that the jejunum, ileum, and ileocolic junction are not grossly identifiable.2 The small intestine is approximately 182 to 198 cm in length, and the large intestine approximately 10 cm. The short length of the intestinal tract results in a gastrointestinal transit time of 3 to 4 hours. The liver is large relative to body weight, making up 4.3% of the body weight in the ferret, as compared with the dog liver, which makes up 3.4% of the dog’s body weight.2 The ferret liver is made up of six lobes: the right lateral, the right medial, the caudate, the quadrate, the left medial, and the left lateral. The pancreas is composed of two limbs: the right, extending along the descending aspect of the duodenum, and the left, situated between the stomach and the spleen. The spleen of the ferret varies greatly in size but normally measures 5.1 cm in length, 1.8 cm in width, and 0.8 cm in thickness.2 It is readily palpable, and when enlarged, may extend from the left cranial abdomen across the midline and down along the right ventral abdomen caudally.
The kidneys of the ferret are bean-shaped, with the right kidney sitting more cranial than the left. The adrenal glands are closely associated with their respective kidneys, both of which are typically embedded in retroperitoneal fat. The left adrenal gland lies medial to the cranial pole of the left kidney, whereas the right adrenal gland may be found under the caudal border of the caudate lobe of the liver and is closely adhered to the caudal vena cava.3 Accessory adrenal tissue has been found in some ferrets.2 This characteristic and the anatomic location of the adrenal glands make complete adrenalectomy a difficult task.
The ferret prepuce is located on the ventral abdomen. The os penis can readily be palpated caudal to the prepucial opening. Hobs also possess a prostate that is situated around the urethra adjacent to the opening of the bladder.2 This is important clinically, as enlargement of the prostate can restrict the flow of urine at this point. The vulva of female is located in the perineal region, ventral to the anus. In anestrus females, the vulva is only a slit-like opening, whereas hormonally active females have a swollen, fleshy vulva that may exhibit a thick, yellow vaginal discharge.
The ferret breeding season is from March to August, although artificial lighting may be used to induce year-round breeding.3 Female ferrets are seasonally polyestrous, induced ovulators. They typically ovulate 30 to 40 hours after copulation. Gestation lasts 41 to 42 days; if fertilization does not occur, a pseudopregnancy may last 41 to 43 days. A physiologic consequence of prolonged estrus in unbred jills is bone marrow toxicity from the chronically elevated estrogen levels.
For further detail on the anatomy of the ferret, see Evans and An.2
HUSBANDRY
Housing
Ferrets may be housed singly or in groups, inside or outside of a house. When kept outdoors, however, they must be protected from extreme weather. Ferrets have difficulty tolerating temperatures above 90° F or below 20° F, and appropriate precautions must be taken to prevent their exposure to these extremes.3 A multilevel wire cage with smooth or wire flooring is appropriate. Glass tanks are not recommended, as they do not provide adequate ventilation. A 24″ ×24″ ×18″ cage is a suitable sized enclosure for a ferret. A cage con-structed out of a nonporous product is ideal for purposes of disinfection.
Nutrition
Ferrets are carnivorous and require a suitable diet. A diet that is high in good-quality animal protein and fat and low in complex carbohydrates and fiber is recommended. An adult, nonbreeding ferret has a dietary protein requirement of 30% to 40% and a fat requirement of 18% to 30%.3 High-quality kitten or commercially prepared ferret food can be offered in a dry pelleted ration. Commercial dog and cat food should be avoided, as these do not have adequate protein levels and often contain plant-based proteins. The ingestion of a diet consisting of plant proteins can result in urinary calculi (see Nutritional Disease). There are several good-quality, commercially prepared ferret diets available, such as Marshall Farms Premium Ferret Diet (Marshall Pet Products, Wolcott, NY), Totally Ferret (Performance Foods, Inc., Broomfield, CO), Mazuri Ferret Diet (Purina Mills, Inc., Richmond, IN, and Zupreem Premium Ferret Diet (Premium Nutritional Products, Inc., Mission, KS). Fat may be supplemented through the use of Linatone (Lambert Kay, Cranbury, NJ), a commercially available fatty acid supplement. Treats may consist of pieces of meat or meat baby food. The sick ferret can be supplemented with Prescription Diet Canine/Feline a/d (Hill’s Pet Products, Topeka, KS), Nutri-Cal (EVSCO Pharmaceuticals, Buena, NJ), or Glucerna (Abbott Laboratories, Columbus, OH), which are high-calorie, high-fat nutritional supplements. (See Therapeutics for further details on nutritional supplementation for the ill patient.) Ferrets that are prone to develop gastric hairballs can be given 1 to 2 ml of a cat laxatone 2 to 3 times a week as a prophylaxis. Water should always be made readily available. This can be accomplished via a heavy bowl or a sipper bottle. Fasting of ferrets for procedures requiring sedation or blood tests may be accomplished by withholding food for 4 to 6 hours. Extended fasts should be avoided, particularly in ferrets that have been diagnosed with insulinoma, to prevent severe hypoglycemia.
PREVENTIVE MEDICINE
Routine Exams
Owners should be educated early on about ferret preventative health care. It begins at an early age before the ferret even leaves the breeding farm with its initial vaccinations. It then becomes the owner’s responsibility to complete the young ferret’s early preventative health care, which consists of vaccination, heartworm prevention, and parasitic identification and treatment. These early visits to the veterinary clinic are great opportunities for veterinarians to educate owners about proper diet and husbandry. Owners should be encouraged to return annually for routine exams and subsequent vaccinations. The routine exam also creates opportunities for grooming (e.g., nail trimming) and identification and scheduling of necessary dental care. As the ferret ages, routine examination and even blood work, such as a complete blood count (CBC) and serum biochemistry panel, become important for the early detection of common, age-related diseases (e.g., insulinoma).
Vaccination
Ferrets are routinely immunized against canine distemper virus (CDV) and rabies virus. Ferrets are quite susceptible to CDV and there is a 100% mortality rate in unvaccinated ferrets infected with CDV.6,7 There is little information available regarding natural rabies infection in the ferret; however, experimental studies have shown ferrets to be susceptible to infection with the virus.8,9 Therefore, it is recommended to immunize them against rabies, particularly in rabies-endemic areas. As in dogs and cats, vaccination in ferrets is initiated in the presence of maternal antibodies and carried out to 14 to 16 weeks of age when maternal antibodies have waned and the ferrets’ own immune system can be stimulated to develop protective antibodies. A recommended schedule for administration of the CDV vaccine is when the ferret is 6 to 8 weeks, 10 to 12 weeks, and 14 to 16 weeks of age; the rabies vaccine should be administered at the time of the last distemper vaccination. Thereafter, annual booster vaccines for CDV and rabies are recommended. The vaccines can be administered subcutaneously in the interscapular region of the body. Some municipalities have passed ordinances requiring intramuscular administration of the rabies vaccine.
A variety of CDV vaccines have been used in ferrets, including several types of modified-live virus vaccines and recombinant canary pox-vectored vaccines, but currently there are only two USDA-approved distemper vaccines for use in ferrets.6–810 The first is a modified-live avian cell culture CDV vaccine, Fervac-D (United Vaccines, Inc., Madison, WI), and the other is a recombinant canary pox-vectored, subunit vaccine, Pure-vax (Merial, Duluth, GA). Canine combination vaccines or modified-live vaccines of ferret cell or low-passage canine cell origin should never be used in ferrets because of the occurrence of vaccine-induced distemper and death, particularly in sick or immunocompromised individuals.6,8
An inactivated rabies vaccine, Imrab (Rhone Merieux Inc., Athens, GA), is licensed for use in ferrets. There are differing regulations on rabies vaccination in ferrets within city, state, and local governments, and it is advisable for the veterinarian to contact these agencies for the current policies. At this time, the American Veterinary Medical Association policy (Model Rabies Control Ordinance, http://www.avma.org/issues/policy/rabies_control.asp; March 17, 2006) for ferrets regarding vaccination, management of animals that have bitten humans, and exposure to rabid or suspect rabid animals is the same as that for dogs and cats.
Adverse reactions of the ferret to vaccination have been reported.6,11,12 Anaphylactic reactions have been documented after ferrets received the Fervac-D vaccine and the Imrab vaccine, both in combination and alone.6 Most of the ferrets experiencing an anaphylactic reaction did so after receiving the CDV vaccine, although there have been reports of reactions occurring in animals after administration of the rabies vaccine alone.6,12 Currently there are no published reports of anaphylactic reaction with the use of the Pure-vax vaccine; however, there are anecdotal reports of similar anaphylactic reactions occurring. The specific component of these vaccines or which vaccine should be implicated as the cause of anaphylactic reactions in ferrets is still unknown.
Anaphylactic reactions to vaccination generally develop within 30 minutes of the injection. Clinical signs associated with a reaction include vomiting, diarrhea, hematochezia, generalized hyperemia (most evident on the nose, mucous membranes, and footpads), hypersalivation, and fever.6,8,12 Less commonly, respiratory distress can develop. Treatment for a vaccine reaction should consist of dexamethasone (2 mg/kg SC), diphenhydramine (1 mg/kg SC or 0.5 mg/kg PO), and epinephrine (0.1 ml of a 1 : 1000 solution SC).6 Supplemental oxygen should be administered as needed for dyspnea. Because anaphylactic reactions can persist for periods up to 24 to 48 hours, prolonged monitoring is recommended, with subsequent treatment with corticosteroids, antihistamines, or epinephrine as needed.12 At the time of vaccination, owners should be advised to wait for 30 minutes after vaccination to monitor for the development of this type of reaction.
A recent report has suggested an association of the development of fibrosarcomas in ferrets with the vaccination site.11 This is the first report of such an association in an animal other than the cat. Examination of fibrosarcomas that developed at the site of vaccination in ferrets revealed similar histologic, immunohistochemical, and ultrastructural characteristics to those reported in feline vaccine-associated sarcomas.
The projected benefits of vaccination must outweigh the adverse reactions in order for them to be considered a useful tool in preventative health care. As a whole, vaccination plays a vital role in reducing the level of infectious disease transmission in a population. However, due to the occurrence of adverse reactions, recommendations for vaccination must be made on an individual basis with the animal’s age, immune status, and current health status in mind. Ferrets that have exhibited previous vaccine reactions should either receive no further CDV vaccine or receive a dose of diphenhydramine 15 minutes before vaccination, although there have been variable results with the latter option. An effective alternative CDV vaccine, which has not yet been found to elicit anaphylactic reactions, is Galaxy-D (Schering-Plough Animal Health Co., Omaha, NE), a modified-live primate cell culture CDV vaccine.7 Duration of immunity induced by this vaccine in the ferret remains unknown, and use of Galaxy-D is extra-label in the ferret, requiring informed owner consent.
Disinfection
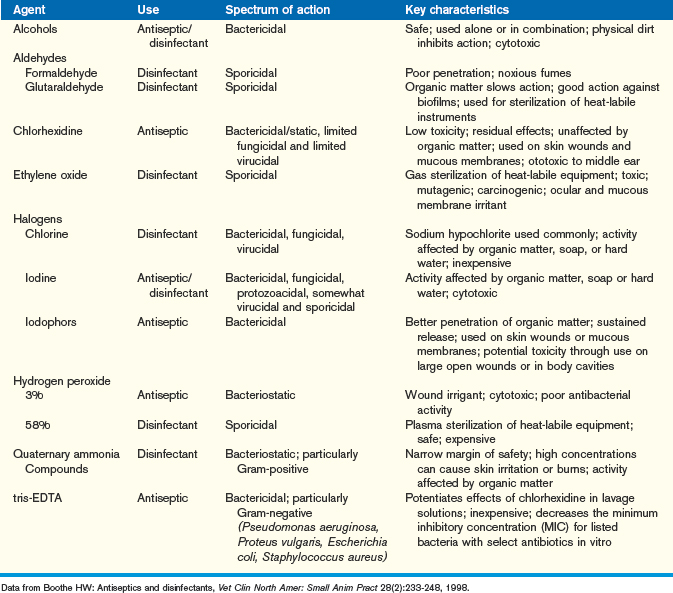
Routine disinfection in the veterinary clinic is important in preventing disease transmission and treating infection. Disinfection involves the killing of pathogenic microorganisms through physical or chemical means. Disinfectants are those chemical agents that are used on inanimate objects such as cages or feeding utensils, whereas antiseptics are those substances that may be used on living tissue for the treatment of open wounds or surgical scrub of the skin or mucous membranes. Sterilization is the complete removal of all living microorganisms from an object, an important application for instruments used for surgery or open wounds where there is a risk of infection. Techniques and agents should be chosen based on the nature and composition of the surface to be disinfected, the level of contamination and the degree of microbial killing desired, and the efficacy and safety of the disinfectant.13 Sterilization should be performed on all surgical instruments through physical (moist or dry heat) or chemical (glutaraldehyde, ethylene oxide, 58% hydrogen peroxide) means. When using a disinfectant, veterinarians should always follow the manufacturer’s recommendations on preparation and use to ensure efficacy and safety. Physical cleaning of any objects intended for disinfection improves the action of the disinfectant, as the killing power of disinfectants is significantly affected by the presence of organic matter. Antiseptics should be chosen based on their spectrum of action, residual effects, and tissue safety. Refer to Table 13-1 for information on commonly used antiseptics and disinfectants.
RESTRAINT
Physical Restraint
Ferrets can be gently grasped under the thorax, allowing the caudal part of the body to rest in the opposite hand or hang freely. Fairly tractable animals can be restrained against the exam table with one hand over the neck and shoulders and the other hand over the hindquarters. With the animal in such a position, eyes, ears, nose, and skin can easily be examined, and vaccinations can be administered. More controlled restraint involves scruffing, or the grasping of the excess skin on the back of the neck, and allowing the caudal half of the body to hang freely. This technique of restraint is great for abdominal palpation. Scruffing often elicits a characteristic yawn from the ferret, aiding examination of the oral cavity or administration of oral medications. Another form of manual restraint involves supporting the length of the ferret’s body along the handler’s forearm while pressing its body snugly against the handler’s body with the head of the ferret directed behind the handler’s body.4 This technique allows for easy administration of injections in the rear of the ferret’s body. Alternatively, feeding the ferret a small amount of Nutri-Cal during a procedure can distract the ferret patient, aiding in restraint.
Chemical Restraint
When physical restraint is not sufficient to safely control the ferret for procedures, chemical immobilization can be a safe alternative. A variety of injectable drugs have been used alone or in combination for restraint or prolonged anesthesia, including ketamine, xylazine, acepromazine, medetomidine, diazepam, and Telazol (tiletamine and zolazepam; Fort Dodge Animal Health, Ft. Dodge, IA) (Table 13-2). Yohimbine and atipamizole are typically used for the reversal of sedation induced by xylazine and medetomidine, respectively. Chemical immobilization using any of these drugs should be restricted to healthy ferrets and avoided in sick and debilitated animals. Additionally, the use of any of these drugs alone is better suited for restraint to perform noninvasive procedures such as blood collection, whereas the ketamine combinations may be adequate for minor surgical procedures.
TABLE 13-2 Dosages for Common Injectable Sedatives, Analgesics, Preanesthetics, Anesthetics, and Antagonists Used in Ferrets
| Drug | Dosage | Use |
|---|---|---|
| Acepromazine | 0.2-0.5 mg/kg SC, IM | sedative |
| 0.1-0.25 mg/kg SC, IM | preanesthetic | |
| Atipamizole | 0.4 mg/kg IM | alpha-2 antagonist |
| Buprenorphine | 0.01 mg/kg SC, IM | analgesia |
| Butorphanol | 0.05-0.1 mg/kg SC, IM | analgesia |
| Diazepam | 1-2 mg/kg IM | sedative |
| Ketamine | 10-20 mg/kg IM | sedative |
| 30-60 mg/kg IM | anesthetic | |
| Ketamine/diazepam | 25-35 mg/kg (K), 2-3 mg/kg (D) IM | anesthetic |
| Ketamine/medetomidine | 5 mg/kg (K), 0.08 mg/kg (M) IM | anesthetic |
| Ketamine/medetomidine/butorphanol | 5 mg/kg (K), 0.08 mg (M), 0.1 mg/kg (B) IM | anesthetic |
| Ketamine/xylazine | 20-40 mg/kg (K), 1-4 mg/kg (X) IM | anesthetic |
| Medetomidine | 0.08-0.1 mg/kg IM | sedative |
| Medetomidine/butorphanol | 0.08 mg/kg (M), 0.1 mg/kg (B) IM | anesthetic |
| Naloxane | 0.04-1.0 mg/kg IV, IM, SC | opioid antagonist |
| Telazol | 12-22 mg/kg IM | anesthetic at higher dosage |
| Xylazine | 1 mg/kg SC, IM | sedative |
| Yohimbine | 0.5 mg/kg IM | alpha-2 antagonist |
Dosages acquired from Marini RP, Fox JG: Anesthesia, surgery, and biomethodology. In Fox JG, editor: Biology and Diseases of the Ferret, ed 2, Philadelphia, 1998, Lippincott Williams and Wilkins.
A ketamine/xylazine combination provides acceptable analgesia, muscle relaxation, duration, and a smooth recovery.14 However, it is important to remember that this combination of drugs has been associated with cardiac arrhythmias and therefore should not be used in ferrets with cardiac disease. Use of the ketamine/xylazine combination also reduces certain hematologic parameters below baseline. The red blood cell count may be reduced by as much as 21%, hemoglobin concentration by 24%, hematocrit by 23%, and plasma protein by 15%. It has been suggested that these alterations may be due to sequestration of the blood in the spleen. Ketamine or Telazol may be used alone for restraint, but it is important to remember that these drugs may not lead to complete analgesia and muscle relaxation.15 Both of these drugs also result in hypersalivation; therefore, the use of an anticholinergic such as atropine is beneficial, particularly when these drugs are used as preanesthetic agents. A medetomidine/ketamine/butorphanol combination provides prolonged anesthetic periods with acceptable analgesia and may be effectively reversed with atipamizole.14
The ideal choice for chemical immobilization in ferrets is gas inhalants. The benefit of using gas inhalants, such as isoflurane or sevoflurane, is that they are safe enough to use in healthy or sick ferrets, and the availability of these gas inhalants in most practices makes immobilization by gas anesthesia a convenient choice. Ferrets may be masked down or induced in a clear gas chamber. When utilizing either of these inhalants, the depth of anesthesia can be quickly adjusted by altering the concentration of the inspired gas. Sevoflurane has shown to provide better systolic arterial pressures and a lower heart rate than isoflurane.14 Additionally, isoflurane can alter hematologic parameters by decreasing the hematocrit, hemoglobin concentration, and red blood cell count by 30% to 38%, and the plasma protein by 20% to 26%, likely by the same mechanism as the ketamine/xylazine combination. More detailed information on anesthesia of the ferret may be found in the Anesthesia section of the chapter.
PHYSICAL EXAMINATION
The physical exam of the ferret should begin even before the animal is handled. Careful observation of the ferret’s mentation, posture, and ambulation can be done while taking a history from the owner. Healthy ferrets often exhibit curiosity for the new environment, actively resisting restraint to explore the new surroundings. Ferrets with underlying disease may present with decreased activity, lethargy, or may even be recumbent. Assessment of body condition can be done at this time as well, taking note of any fluctuations in body weight with each visit. When this initial assessment is completed, the ferret can be minimally restrained for a basic exam. The body temperature should be taken before the start of the exam to prevent elevated recordings from increased body heat secondary to any struggle that may occur before obtaining the reading. Ferrets readily object to insertion of a rectal thermometer and restraint will be necessary. The normal body temperature range of a ferret is between 100° F and 104° F.8
Palpation of the ferret abdomen can be done with relative ease and can yield important information about the patient’s condition. The ferret should be held either with one hand under the thorax or by scruffing the neck while the other hand is used to palpate the abdomen. Palpation of the j-shaped stomach in the cranial abdomen should be performed carefully in ferrets presenting for anorexia or vomiting, as pain or further trauma may be elicited in animals presenting with foreign body obstruction. Sometimes the foreign body itself may be palpable as well. The spleen can be found in the left cranial abdomen. It is often enlarged, extending caudoventrally across the abdomen with the apex along the right side of the body. Enlargement of the spleen can occur in clinically healthy ferrets, but an irregular shape or masses on the spleen are considered abnormal and should be investigated further. The intestines are palpated for the presence of gas or thickened bowel loops. The mesenteric lymph nodes are also palpated for enlargement. An attempt should be made to palpate the adrenal glands lying medial to both kidneys, particularly in ferrets presenting with signs of adrenal gland disease. Normal adrenal glands typically cannot be palpated, and in obese ferrets it may be difficult to palpate enlarged glands because of the presence of retroperitoneal fat; however, significantly enlarged adrenal glands may be detected on palpation. The bladder can be palpated in the caudal abdomen and may be painful in ferrets that have urethral obstruction and/or bladder stones. Male ferrets presenting with a distended bladder due to urethral blockage will likely require drainage of the bladder before the prostate can be assessed. An enlarged prostate may be palpated caudal to the bladder in males with adrenal disease.
In male ferrets, the prepuce should be examined for erythema or swelling that may be associated with blockage of the distal urethra. The testicles of intact males should be examined and palpated, as testicular neoplasia can occur in the hob.16 The vulva of jills should always be checked for swelling or discharge, a sign of estrus in intact ferrets and a sign of adrenal gland disease or remnant ovarian tissue in spayed females.
CLINICAL TECHNIQUES AND DIAGNOSTIC TESTING
Venipuncture and Hematologic Testing
Venipuncture is an important clinical technique that can be done with or without sedation. There are several sites for venipuncture that may be utilized depending on the volume of blood required for testing and the availability of assistants for restraint. Blood is assumed to be 5% to 7% of the ferret’s body weight, and 10% of the ferret’s blood volume may be safely withdrawn during a single collection.17 Diagnostic laboratories performing hematologic tests on ferrets typically require only 0.5 to 2 ml of blood, whereas larger quantities are needed from ferrets serving as blood donors.
Larger quantities of blood should be withdrawn from the jugular vein or anterior vena cava. Again, the ferret may be restrained like a cat for jugular venipuncture, with the forelegs pulled forward from the body and the head extended, nose pointed upward. The jugular veins of the ferret lie more laterally than the cat. Overextension of the head should be avoided as this can reduce jugular vein distention. Another technique for jugular venipuncture involves positioning the towel-wrapped ferret into dorsal recumbency with its head extended.14 While the ferret is distracted with Nutri-Cal, venipuncture can be performed using a 22- or 23-gauge needle bent at a 30-degree angle, attached to a 3-ml syringe. When collecting blood from the anterior vena cava, the veterinarian should consider sedating active ferrets with general anesthesia. This site should not be used in ferrets with bleedings disorders, because this vein is located within the thoracic cavity and does not allow for direct hemostasis by the clinician. With the ferret placed in dorsal recumbency, a 22- or 23-gauge needle, attached to a 1-to-6-ml syringe, is inserted at the junction of the first rib and the sternum (Figure 13-1). If you approach from the animal’s left side, the needle should be directed toward the right elbow. If you approach from the animal’s right side, the needle should be inserted parallel to the sternum. The anterior vena cava is superficial within the thoracic cavity and may often be located upon retraction of the needle. Always maintain a negative pressure draw, looking for a blood “flash” in the hub of the needle.

Figure 13-1 With the anesthetized ferret in dorsal recumbency, the anterior vena cava can safely be sampled.
Diagnostic laboratories usually establish reference ranges for the tests offered for ferrets. Reference ranges for the ferret CBC and serum chemistry panel may be found in Box 13-3 and Table 13-3, respectively. Hematologic parameters for the ferret are very similar to those of the cat; however, the erythrocyte, hematocrit, and reticulocyte counts are generally higher in the ferret.18 Ferrets also have a slower erythrocyte sedimentation rate; therefore, it is necessary to spin microhematocrit tubes for longer time periods than for dogs or cats. There is little information available on blood coagulation parameters in ferrets, although the prothrombin time has been reported to be 14.4 to 16.5 seconds.18 As with other animals, there is an age-related decrease in the enzyme alkaline phosphatase due to a reduction in the bone isozyme when rapid growth has ceased.18 Differential diagnoses for ferret patients that present with elevated liver enzymes include liver disease, exposure to ingested or environmental toxins, and Aleutian disease.19 Creatinine is normally cleared from the blood much faster in ferrets than other mammalian species; thus, any elevation in creatinine is significant and should be pursued further.20
BOX 13-3 Hematologic Values for the Ferret*
| Value | Female | Male |
|---|---|---|
| PCV (%)18–20 | 34.6-55 | 33.6-61 |
| Hemoglobin (g/dl)18–20 | 11.9-17.4 | 12-18.5 |
| Erythrocytes (×106/mm3)18,19 | 6.77-9.76 | 7.1-13.2 |
| Reticulocytes (%)18,19 | 2-14 | 1-12 |
| Platelets (×103/mm3)18,19 | 264-910 | 297-730 |
| MCV (fl)18 | 44.4-53.7 | 42.6-52.5 |
| MCH (pg)18 | 16.4-19.4 | 13-7.19-7 |
| MCHC (g/dl)18 | 33.2-42.2 | 30.3-34.9 |
| Leukocytes (×103/mm3)18–20 | 2.5-18.2 | 1.7-19.1 |
| Neutrophils (%)18–20 | 12-84 | 11-82 |
| Bands (%)18,20 | 0-4.2 | 0-2.2 |
| Lymphocytes (%)18–20 | 12-95 | 12-73 |
| Monocytes (%)18–20 | 1-8 | 0-9 |
| Eosinophils (%)18–20 | 0-9 | 0-8.5 |
| Basophils (%)18–20 | 0-2.9 | 0-2.7 |
* Combined values for adult female ferrets and for adult intact and castrated males from Fox JG: Normal clinical and biologic parameters. In Fox JG, editor: Biology and Diseases of the Ferret, ed 2, Philadelphia, 1998, Lippincott Williams and Wilkins; Thornton PC, Wright PA, Sacra PJ et al: The ferret, Mustela putorius furo, as a new species in toxicology, Lab Anim 13 : 119-124, 1979; and Lee EJ, Moore WE, Fryer HC et al: Hematological and serum chemistry profiles of ferrets (Mustela putorius furo), Lab Anim 16 : 133-136, 1982.
TABLE 13-3 Serum Biochemical Values for the Ferret*
| Value | Female | Male |
|---|---|---|
| Sodium (mmol/L)18–20 | 142-156 | 137-162 |
| Potassium (mmol/L)18–20 | 4.2-7.7 | 4.1-7.3 |
| Chloride (mmol/L)18–20 | 112-124 | 102-126 |
| Calcium (mg/dL)18–20 | 8-10.2 | 8.3-11.8 |
| Phosphorus (mg/dL)18–20 | 4.2-10.1 | 4-8.7 |
| Glucose (mg/dL)18–20 | 85-207 | 62.5-198 |
| Urea nitrogen (mg/dL)18–20 | 10-45 | 11-42 |
| Creatinine (mg/dL)18–20 | 0-1 | 0.2-1.6 |
| Protein (g/dL)18–20 | 5.1-7.2 | 5.3-7.4 |
| Albumn (g/dL)18–20 | 2.6-4.1 | 2.8-4.2 |
| Globulin (g/dL)18 | 2.2-3.2 | 2.0-4.0 |
| A/G ratio (g/dL)18 | 1.0-1.6 | 0.8-2.1 |
| Total bilirubin (mg/dL)18,19 | 0-1.0 | 0-0.1 |
| Cholesterol (mg/dL)18,19 | 122-296 | 64-221 |
| Alkaline phosphotase (U/L)18–20 | 3-62 | 11-120 |
| Aspartate aminotransferase (U/L)18,19 | 40-120 | 28-248 |
| Alanine aminotransferase (U/L)18,20 | 54-280 | 54-289 |
| Carbon dioxide (mmol/L)18,20 | 16.5-27.8 | 12.2-28 |
* Combined values for female ferrets and intact and castrated male ferrets are from Fox JG: Normal clinical and biologic parameters. In Fox JG, editor: Biology and Diseases of the Ferret, ed 2, Philadelphia, 1998, Lippincott Williams and Wilkins; Thornton PC, Wright PA, Sacra PJ et al: The ferret, Mustela putorius furo, as a new species in toxicology, Lab Anim 13 : 119-124, 1979; and Lee EJ, Moore WE, Fryer HC et al: Hematological and serum chemistry profiles of ferrets (Mustela putorius furo), Lab Anim 16 : 133-136, 1982.
Urine Collection and Urinalysis
Catheterization of the ferret usually requires general anesthesia. Sterile technique is recommended to prevent the iatrogenic introduction of contaminants into the urinary tract. Again, male ferrets can be difficult to catheterize; this is because of the acute angle at which the urethra turns at the ischial arch. The presence of a urethral stone or prostatomegaly may make the procedure impossible without causing additional trauma. Care should be taken to prevent iatrogenic perforation of the urethra when passing the catheter through this turn at the ischial arch. With the ferret placed in dorsal recumbency, the penis is extracted from the prepuce, initiating the catheterization process. Magnification may be necessary to visualize the urethral opening on the ventral aspect of the penis. A needle with the bevel filed off can be inserted into the opening to flush and distend the urethra with saline to facilitate the introduction of the catheter into the urethra. A water-soluble lubricant also makes passage of the catheter easier. A 20- to 22-gauge, 8-inch jugular catheter, a 3.5-Fr red rubber tube, or a 3.0-Fr Slippery Sam Tomcat urethral catheter (Cook Veterinary Products, Eight Mile Plains, Queensland, Australia) may be used.21 The latter option is preferred based on the size and rigidity of the catheter. An intravenous catheter stylet or sterile guitar string can be used to provide rigidity to the catheter; again, the veterinarian needs to be conscious not to traumatize the urethra during the passage of the catheter.21
Female catheterization is an easier technique. With the animal under general anesthesia, the female should be placed in ventral recumbency with the caudal body elevated. The urethral opening is located on the ventral floor of the vaginal vestibule, just cranial to the clitoral fossa. It may be visualized with an otoscope, endoscope, or a vaginal speculum. The catheter can be placed by sliding it along the ventral floor of the vagina, directed at an acute angle ventrally, until it enters the urethra.21,22 Catheters that are placed for therapeutic purposes can be secured by suturing butterfly tape to the skin, and if necessary, catheters can be attached to a urine collection system. Ferrets should be closely monitored for entanglement of the tubing or disturbance of the catheter set.
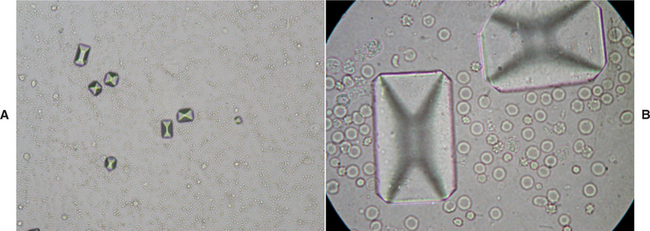
Normal ferret urinalysis parameters can be found in Table 13-4. Protein is a common finding in ferrets, possibly due to high systolic pressure and thicker intrarenal arterial walls.18 With this finding, however, the clinician should include genitourinary infection and Aleutian disease as differential diagnoses. Blood in the urine may be the result of iatrogenic trauma to the urethra during catheterization, cystitis, urethritis in males, or estrus in females. False positives for ketonuria in male ferrets have been reported with the use of urinalysis strips. The similarity in color of the dark urine produced by male ferrets and the reagent test strip is a possible explanation of the ketonuria false positive result.17 Examination of urine sediment may reveal crystalluria in patients with blocked urethras or an accumulation of white blood cells in ferrets with urinary tract infection (Figure 13-2). An elevated urine pH has been associated with a diet high in plant proteins and is a good indication that diet correction may prevent crystalluria and subsequent urolithiasis.
TABLE 13-4 Normal Urinalysis Parameters for the Ferret
| Parameter | Males | Females |
|---|---|---|
| Volume (ml/24 hr) | 8-48 | 8-140 |
| pH | 6.5-7.5 | 6.5-7.5 |
| Protein (mg/dl) | 7-33 | 0-32 |
| Potassium (mmol/24 hr) | 1.0-9.6 | 0.9-5.4 |
| Sodium (mmol/24 hr) | 0.4-6.7 | 0.2-5.6 |
| Chloride (mmol/24 hr) | 0.7-8.5 | 0.3-7.8 |
Data collected from Fox JG: Normal clinical and biologic parameters. In Fox JG, editor: Biology and Diseases of the Ferret, ed 2, Philadelphia, 1998, Lippincott Williams and Wilkins.
Fecal Examination
Although intestinal parasitism is relatively uncommon in captive ferrets, the examination of feces as a diagnostic tool should not be overlooked considering the susceptibility of the ferret to a variety of gastrointestinal (GI) disorders. Due to the short GI transit times and the habit of ferrets to defecate in the same location within their cages, acquisition of a sample is not difficult. Often, restraint in the exam room is enough to stimulate defecation by the ferret patient. The color, quantity, and consistency of the fecal sample should be described in the record, as this may change according to the disease process present (e.g., green, mucoid diarrhea associated with green slime disease). Fecal exams for parasite identification may be performed at the time of the annual exam using a direct smear and a fecal flotation technique with sodium nitrate or a sugar solution. Further discussion of internal parasitism can be found in the section on infectious diseases. Ferrets that present with diarrhea should also have a fecal Gram stain done to evaluate the sample for the presence of pathogenic microorganisms. If bacterial pathogens are suspected, a fecal culture should be pursued.
Diagnostic Imaging
Ultrasonography in the ferret follows the same principles applied to the cat and dog. Although ultrasonography does not require sedation, a technique of distraction (e.g., Nutri-Cal treat) usually reduces any active resistance. A higher frequency transducer, such as a 10- or 11-MHz transducer, is recommended to enhance the resolution of the image for the smaller ferret patient.23
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree