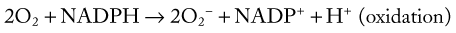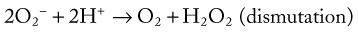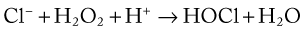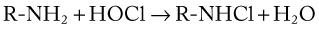Chapter 5 Evaluation of Leukocytic Disorders
Leukocyte Types and Numbers in Blood
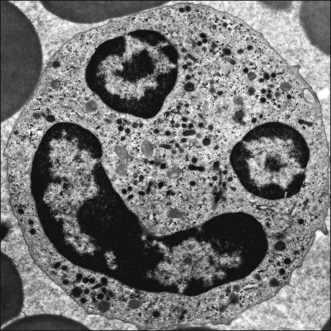
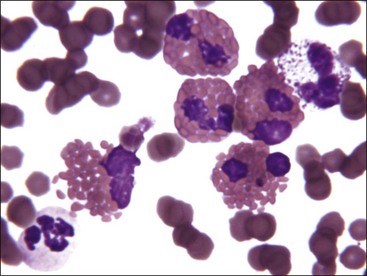
Mammalian leukocytes or white blood cells have been classified as either polymorphonuclear leukocytes (PMNs) or mononuclear leukocytes. The PMNs have condensed, segmented nuclei. They are commonly referred to as granulocytes because they contain large numbers of cytoplasmic granules (Fig. 5-1). The term granulocyte is preferred in veterinary medicine because nuclear segmentation does not occur in the granulocytes of most reptiles and it is not as prominent in birds as it is in mammals. The granules in these cells are lysosomes containing hydrolytic enzymes, antibacterial agents, and other compounds. Primary granules are synthesized in the cytoplasm of late myeloblasts or early promyelocytes. They appear reddish purple when stained with routine blood stains such as Wright-Giemsa (see Chapter 8). Secondary (specific) granules appear at the myelocyte stage of development in the bone marrow. Three types of granulocytes (neutrophils, eosinophils, basophils) are identified by the staining characteristics of their secondary granules (Fig. 5-2).
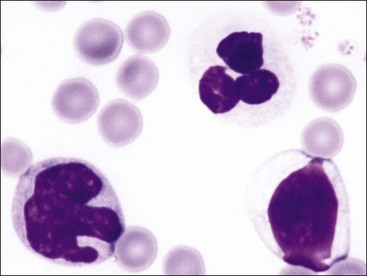
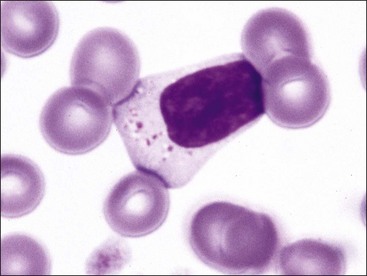
Mononuclear leukocytes in blood are classified as either lymphocytes or monocytes (Fig. 5-3). These cells are not devoid of granules but rather have lower numbers of cytoplasmic granules than do granulocytes. Lymphocytes have high nuclear-to-cytoplasmic (N : C) ratios. Their nuclei have coarsely clumped chromatin and are usually round, but they may be oval or slightly indented. A low percentage of lymphocytes in blood have focal accumulations of red- or purple-staining granules within the cytoplasm (Fig. 5-4). These granular lymphocytes may be cytotoxic T lymphocytes or natural killer (NK) cells. Monocytes are usually larger than lymphocytes, have nuclei with finer chromatin clumping that are more variable in shape (round-, kidney-, or band-shaped), and have N : C ratios of 1 or less. They often exhibit cytoplasmic vacuoles in films prepared from blood collected with an anticoagulant.
The total number of leukocytes varies considerably by species. Among common domestic animals, the mean total leukocyte count is highest in pigs (16,000/µL) and lowest in cattle and sheep (8000/µL).226 Neutrophils and lymphocytes are the most numerous leukocyte types present in the blood of healthy domestic mammals. Dogs, cats, and horses usually have more neutrophils in blood than lymphocytes. In contrast, lymphocytes are usually more numerous in pigs, cattle, sheep, goats, and rodents.226 Numbers of neutrophils and lymphocytes change with age after birth. The neutrophil-to-lymphocyte ratio tends to be higher at birth than in later life, in part because of the increased blood cortisol concentration at birth.84,249,312,331,460 Cortisol causes circulating neutrophil numbers to increase and circulating lymphocyte numbers to decrease. Calves have neutrophil-to-lymphocyte ratios well above 1.0 at birth owing to neutrophil numbers above and lymphocyte numbers below those of adults. Within a week, neutrophil numbers decrease and lymphocyte numbers increase to counts that are approximately equal, with lymphocyte counts above neutrophil counts by 3 weeks of age.249,331 Low numbers of monocytes, eosinophils, and basophils are present in normal mammals. Basophil numbers are especially low in dogs and cats, with none being seen in blood from many healthy animals.
Leukocyte Kinetics
In contrast to erythrocytes, leukocytes do not exhibit a life span in blood but rather leave blood at random times in response to chemoattractant stimuli. Except for lymphocytes that recirculate, it was thought that leukocytes did not reenter the circulation after migration into the tissues. However, there is evidence that some tissue eosinophils return to the circulation via lymphatics478 and that, in the absence of inflammation, some monocytes may shuttle back to the bone marrow.506
Neutrophils
Following release from the bone marrow, neutrophils are normally present in blood for a short time (half-life about 5 to 10 hours) before they egress into the tissues.75,116,383,433 Neutrophils appear to survive no more than a few days in tissues.433 Neutrophils that remain in blood spontaneously undergo apoptosis and are removed by macrophages in the spleen, liver, and bone marrow via a phagocytic process (termed efferocytosis) that does not generate an inflammatory reaction.162,328,422
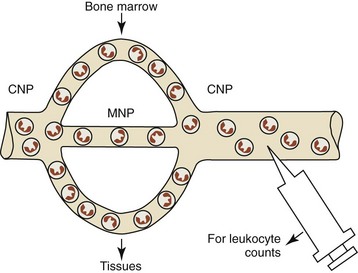
Neutrophils occur in circulating and marginating pools in blood, with 50% or less of the total blood neutrophil pool being present in the circulating pool (Fig. 5-5).226,433 The circulating neutrophil pool (CNP) is assessed by routine blood sample collection. The marginating neutrophil pool (MNP) consists of neutrophils that are transiently retained in capillaries and veins. The lung has long been considered the predominant organ contributing to the MNP121,309; however, recent studies suggest that the liver, spleen, and bone marrow may contribute substantially to the MNP.445 The retention of neutrophils in the vasculature does not appear to involve neutrophil adhesion to endothelial cells.260 The size of the MNP in an organ is related to its blood flow and the neutrophil intravascular transit time through the organ.445 Neutrophil margination is increased in lungs when blood flow is reduced and decreased when blood flow is increased.455 The absolute neutrophil count measured in blood samples can be affected by cell movements between the MNP and CNP. A net movement of neutrophils from the MNP to the CNP increases the circulating blood neutrophil count. A net movement in the opposite direction results in a decreased circulating blood neutrophil count.435
Eosinophils and Basophils
The half-life reported for human eosinophils ranges from 8 to 18 hours.368 Little information is available concerning blood basophil kinetics, but a half-life of 2 to 3 days has been reported for humans.440 Basophils appear to survive no more than a few days in tissues.164 In contrast, eosinophils may remain in tissues for weeks to months unless they migrate into airways or the gastrointestinal tract.478
Monocytes
The half-life for monocytes in blood is reported to vary from 0.5 to 3 days in rabbits, mice, and humans.185,506 Monocytes also have a marginating pool within pulmonary capillaries.121 Monocytes develop into macrophages and dendritic cells in the tissues, where they survive for variable time periods.506 Macrophages may survive up to 3 months in tissues.165 Dendritic cells in lymphoid organs are reported to survive 10 to 14 days.284 In contrast, dendritic cells in skin (Langerhans cells) are reported to survive more than a year.322
Lymphocytes
Most lymphocytes reside within lymphoid organs (lymph nodes, thymus, spleen, and bone marrow). Only about 2% to 5% of lymphocytes circulate in blood.50,458 Like neutrophils and monocytes, lymphocytes have a MNP within pulmonary capillaries.121,455 Depending on the species and individual variability, about 50% to 75% of blood lymphocytes are T lymphocytes and about 10% to 40% are B lymphocytes. NK cells account for 5% to 10% of blood lymphocytes.458 Some NK cells appear as granular lymphocytes, but not all. A subset of CD8+ T lymphocytes also appear as granular lymphocytes.33
A majority of blood lymphocytes are naive T and B lymphocytes, and most of the remaining blood lymphocytes are antigen-primed memory T and B lymphocytes.140 The fraction of memory lymphocytes increases and the fraction of naive lymphocytes decreases in the blood of humans as they age.293 Most lymphocytes in blood have come from peripheral lymphoid organs (primarily lymph nodes). Lymphocytes circulate for a short time in blood (half-life about 30 minutes), exit, migrate through lymphoid tissues (and to some degree extralymphoid tissues), and return to blood via lymphatics.50 B and T lymphocytes migrate through lymph nodes with average velocities of around 6 and 12 µm/min, respectively. It is estimated that it takes about 1 day for a recirculating lymphocyte to migrate through a lymph node.40,507 Recirculation allows lymphocytes, with their complete repertoire of unique antigen receptors, to be available for immune reactions throughout the body.
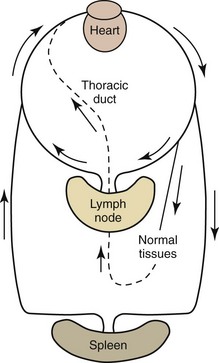
Naive lymphocytes have a propensity to recirculate through lymph nodes. They migrate into lymph nodes through high endothelial venules (HEVs) because these venules have adhesion molecules and chemokines on their surfaces that recognize complementary adhesion and chemokine receptors expressed on naive lymphocyte surfaces.399 Recirculating lymphocytes generally exit the lymph nodes via the efferent lymphatics; however, they appear to emigrate from lymph nodes via paracortical postcapillary venules in pigs.51 Efferent lymphatics join together to form large lymphatic vessels, the largest of which is the thoracic duct, and drain into the blood at the level of the heart (Fig. 5-6).
Dendritic cells are exposed to antigens in tissues and migrate to draining lymph nodes. When naive T and B lymphocytes are exposed to their cognate antigens presented by dendritic cells in lymph nodes, they proliferate and form effector lymphocytes and long-lived memory lymphocytes. Dendritic cells present not only antigens but also environmental signals (including vitamin A and D3 metabolites) that program migration pathways.428 Memory lymphocytes develop an array of adhesion molecules and chemokine receptors that target their subsequent migration to specific tissues including skin, intestinal mucosa, and lungs.50,399 Tissue lymphocytes are picked up by afferent lymphatics and are carried to lymph nodes, where they exit by efferent lymphatics, traverse large lymphatic vessels, and reenter the blood (see Fig. 5-6). Most plasma cells and proliferating lymphocytes, such as those present in germinal centers, do not express the adhesion molecules necessary for migration.458
Lymphocytes generally survive much longer than granulocytes. Naive T lymphocytes are reported to have a half-life of about 40 days in mice. CD8+ memory T lymphocytes and some CD4+ memory T lymphocytes are long-lived in mice (many months), but other CD4+ memory T lymphocytes survive only about 2 months. Memory T lymphocytes are reported to survive for many years in humans.309 Long-term survival may require cell proliferation; therefore it may be a matter of semantics whether long-term survival refers to an individual lymphocyte or a population of lymphocytes.
The t1/2 disappearance rate for B lymphocytes from the recirculating pool is reported to be 2 to 3 weeks in humans. Long-term-memory B lymphocytes appear to be maintained as proliferating clones of cells rather than as individual cells that survive for a long time.293 Plasma cells survive for variable periods of time. Those that develop in sites of inflammation disappear when the inflammation is resolved. However, some plasma cells may survive for years in humans, especially within stromal niches in bone marrow.339
An NK cell’s half-life in the circulation is about 7 to 10 days in mice and 12 days in humans under normal conditions. NK cells leave the circulation by entering tissues during steady-state conditions or through cell death. NK cell survival is promoted by the cytokine, interleukin-15 (IL-15).513 NK cells are located in many organs including spleen, lung, bone marrow, lymph nodes, liver, intestine, skin, and thymus (low numbers). Some NK cells recirculate through blood and lymph.117,187 The spleen and bone marrow appear to provide NK cell reserves that can rapidly enter the blood and subsequently migrate into tissues in response to inflammation. NK cell migration from blood to inflammatory sites occurs by mechanisms similar to those described for granulocytes. These include initial adhesion to endothelial cells via selectins, followed by tighter adhesion to endothelial cells via integrins and chemoattractant-directed migration. Chemoattractants include certain chemokines as well as bacterial products, leukotrienes, and C5a. Marked infiltrates of NK cells are also present in the uterus during pregnancy.187
Leukocyte Functions
Neutrophil Functions
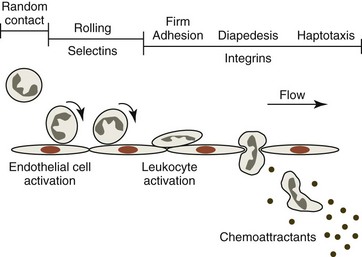
Neutrophils are essential in the defense against invading microorganisms, primarily bacteria. To be effective, they must recognize inflammatory signals, leave the blood, migrate through tissue to a site where bacteria are present, and then neutralize the bacteria. Neutrophils display glycoprotein adhesion molecules on their surfaces that are needed for various adhesion-dependent functions, including adhesion to endothelium and subendothelial structures, spreading, haptotaxis, and phagocytosis.106 Unless they are activated, neutrophils and endothelial cells exhibit little tendency to adhere to each other. Following the stimulation of endothelial cells by mediators—such as thrombin, histamine, and oxygen radicals—P-selectin is rapidly mobilized from storage granules and expressed on the surfaces of endothelial cells. Inflammatory mediators, including interleukin-1 (IL-1) and tumor necrosis factor (TNF), promote the expression of E-selectin adhesion molecules on the surface of activated endothelial cells within 1 to 2 hours.58 These oligosaccharide-binding selectin molecules, acting in concert with the L-selectin adhesion molecule expressed on the surface neutrophils, bind to their counterligands, most notably P-selectin glycoprotein ligand-1 (PSGL-1), which results in the initial adhesion of unstimulated neutrophils to activated endothelial cells (Fig. 5-7).511 As a result of selectin binding between neutrophils and activated endothelial cells, the velocity of neutrophils in the circulation is markedly decreased and they are seen to roll along the endothelium.510
Activated endothelial cells produce factors including interleukin-8 (IL-8) and platelet activating factor (PAF, a biologically active phospholipid) that result in neutrophil activation. Other mediators that can activate neutrophils include opsonized particles, immune complexes, N-formylated bacterial peptides, granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), and chemoattractants produced during inflammation.58 Neutrophil activation results in increased expression and enhanced binding affinity of β2 integrin adhesion molecules and shedding of L-selectin molecules. β2 integrins (CD11a,b,c/CD18) are heterodimers that bind with variable affinity to intercellular adhesion molecules (ICAMs). β2 integrin binding further slows rolling and ultimately results in the firm adhesion of neutrophils to endothelial cells. Adherent (activated) neutrophils then spread and exhibit pseudopod formation. Neutrophil activation also promotes degranulation, superoxide generation, and the production of arachidonate metabolites, to be discussed later.58
In addition to increased β2 integrin affinity, activated neutrophils have increased numbers of surface receptors and/or enhanced receptor affinity for chemoattractants.432 These receptors are also found in granules, suggesting that they are mobilized to the cell surface during neutrophil activation. When they are exposed to chemoattractants, neutrophils penetrate the wall of postcapillary venules, primarily by moving between endothelial cells.58 Less than 5 minutes is required for neutrophils to pass between endothelial cells, but 15 minutes or more may be required for them to pass through the vascular basement membrane.277 Once outside the vessel, the active directional migration of neutrophils in tissues occurs largely by haptotaxis, which means migration up a gradient of immobilized chemoattractants rather than soluble chemoattractants (chemotaxis). A wide variety of substances can function as a chemoattractant, including IL-8 and other chemokines, C5a (complement fragment), leukotriene B4 (a product of arachidonic acid metabolism via the lipoxygenase pathway), PAF (1-0-alkyl-2-acetyl sn-glyceryl phosphorylcholine) and bacterial products (e.g., N-formyl-methionyl oligopeptides) recognized by Toll-like receptors.58,106
During their movement toward increasing concentrations of chemoattractants, neutrophils become elongated, with a frontal pseudopod extending in the direction of movement and a distal uropod that consists of fine trailing appendages. Membrane sheets (lamellipodia) on the leading edge of the pseudopod are in continual motion, orienting in the direction of chemoattractants.441 Neutrophils crawl (10-12 µm/min) toward the source of the chemoattractant by the binding of integrin surface molecules to their respective ligands within the extracellular matrix at the frontal pseudopod and detaching from these ligands at the distal uropod. Although present in only small amounts on the surfaces of circulating blood neutrophils, activated neutrophils express increased surface β1 integrins, which appear to be the most important class of integrins for migration. Members of the β1 integrin family have high affinity for proteins in the extracellular matrix, including collagen, laminin, fibronectin, and vitronectin.280 Movement depends on actomyosin-mediated contractions at the leading edge for pseudopod advancement and in the trailing end to break adhesive contacts.441
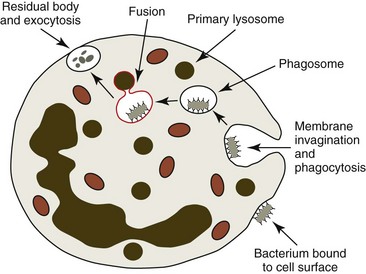
For phagocytosis to occur, neutrophils must be able to first bind invading bacteria to their surfaces (Fig. 5-8). This adherence is greatly potentiated if bacteria have been opsonized (have antibodies and complement components bound to their surfaces) because neutrophils have immunoglobulin Fc and C3b receptors on their surfaces. Following binding, bacteria are engulfed by the neutrophils’ cytoplasmic processes, which extend around the organisms. The membranes of the neutrophils’ cytoplasmic processes fuse to form phagocytic vacuoles surrounding the engulfed bacteria.58,106
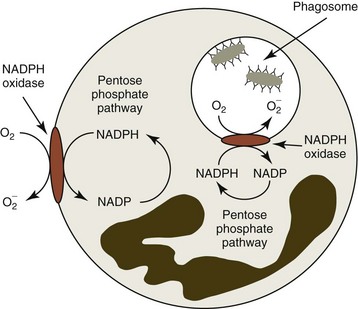
Bacterial killing involves a multitude of mechanisms that are set into motion by two cellular events, initiation of the respiratory burst and degranulation. The respiratory burst is initiated by the activation of an NADPH oxidase enzyme (Fig. 5-9). This enzyme is normally “inactive” in resting or unstimulated phagocytes. Enzyme activation depends on the assembly of multiple components, some of which are already membrane-bound and others that must be translocated from the cytoplasm to the membrane. Activated NADPH oxidase is located in the plasma membrane and becomes incorporated into the phagocytic vacuole. It catalyzes the one-step reduction of O2 to form superoxide (O2−).106 The NADPH needed to generate superoxide is formed in the pentose phosphate pathway. The superoxide thus formed undergoes dismutation to form hydrogen peroxide, as shown below:
Hydrogen peroxide and superoxide can diffuse from the phagocytic vacuole into the cytoplasm of the cell. Activated neutrophils utilize the superoxide dismutase and glutathione peroxidase reactions to protect themselves from these oxidants. The latter reaction requires that additional NADPH be generated to maintain glutathione in the reduced form. Activation of the respiratory burst requires neither phagocytosis nor degranulation to occur. In addition to opsonized particles, chemoattractants, such as C5a, can activate the respiratory burst.106
Superoxide, other free radicals (e.g., hydroxyl radical), and H2O2 may be involved directly in the killing of bacteria, but killing is potentiated by degranulation, which results in the fusion and release of the contents of lysosomal granules into the phagocytic vacuole (see Fig. 5-8). Myeloperoxidase is an iron-containing enzyme located in the primary granules of neutrophils. The myeloperoxidase reaction greatly enhances the bactericidal potency of H2O2. This reaction catalyzes the oxidation of chloride to hypochlorous acid, resulting in halogenation of bacterial cell walls (see below) and loss of integrity.106
Other enzymes are also present in primary and secondary granules of neutrophils.432 These include collagenase, acid and neutral hydrolases, and lysozyme, which hydrolyzes glycosidic linkages in the cell walls of certain bacteria. These enzymes are probably more important in digestion than in killing. Nonenzymatic agents are also involved in neutrophil defense. A number of cationic proteins and peptides in neutrophil granules have antimicrobial properties.432 Of these molecules, defensins appear to be the most common. With the lowest molecular weight, defensins are small (4 kD) antimicrobial peptides within primary granules that act against bacteria and other microorganisms by altering their membrane permeability. They are inserted into the lipid bilayer, disrupting interaction between lipid molecules. In addition, lactoferrin occurs within secondary granules and chelates iron required for microbial growth.432
The growth factors G-CSF and GM-CSF are important, not only in the production of neutrophils but also in promoting neutrophil survival and function. G-CSF and GM-CSF are produced by macrophages, neutrophils, and other cell types at inflammatory sites.137 They prime neutrophils in ways that enhance their functions and inhibit apoptosis of neutrophils at sites of inflammation.106,360 Neutrophils become more adhesive and responsive to other stimuli, including chemoattractants, and their respiratory burst is enhanced after they bind these growth factors.106,137,304,499
Following the killing and digestion of bacteria, the phagocytic vacuole fuses with the plasma membrane and discharges the killed bacteria, products of degraded bacteria, and contents of granules to the outside of the cell in a process called exocytosis (see Fig. 5-8). Discharge of granules can also occur following activation of neutrophils in the absence of phagocytosis. Considerable tissue injury occurs in areas where neutrophils are activated because of the oxidants they produce and the granule contents they release.
Neutrophils can trap and kill bacteria and fungi without phagocytosis by releasing neutrophil extracellular traps (NETs). These NETs consist of fibers (composed of DNA, histones, and proteins from granules) that can kill microbes.158 Initial reports indicated that these NETs were generated by neutrophils undergoing cell death158; however, more recent reports indicate that NET formation can occur without neutrophil death.365,509 NET production is an active process, not the result of leakage during cellular disintegration. The NETs formed by living neutrophils contain mitochondrial DNA but no nuclear DNA.509 In addition to mammalian neutrophils,192,477 neutrophils from fish and heterophils from birds produce NETs when they are appropriately stimulated.85,365
Several pathogens have co-opted phagocytic and killing processes to survive within phagocytes. These include Listeria, Yersinia, and Mycobacterium species.106
Eosinophil Functions
Most tissue eosinophils reside in the gastrointestinal mucosa.335 Eosinophils have less phagocytic ability than neutrophils and provide poor host defense against bacterial or viral agents.207 However, eosinophils are an important component of the type 2 cytokine-induced inflammatory response that is critical in the host defense against helminth infections and is responsible for the pathogenesis of type 1 hypersensitivity allergic reactions. CD4+, type 2 helper T (TH2) lymphocytes secrete a group of cytokines that result in the recruitment and/or activation of effector cells including B lymphocytes producing IgE, mast cells, basophils, and eosinophils.346
Rather than simply responding to an otherwise innocuous environmental antigen by producing IgG or IgA antibodies, some animals respond to environmental antigens with an exaggerated TH2 response that produces excessive amounts of IgE. IL-4 and IL-13 from TH2 lymphocytes and the presence of activated mast cells, basophils, and eosinophils stimulate B lymphocytes to switch to IgE production.381,431 This excess IgE binds to receptors (FcεRI) on mast cells in the tissues and primes the mast cells to bind the environmental antigen (allergen) that stimulated the IgE response. When the primed mast cell encounters the allergen and it cross-links two of the bound IgE molecules, the mast cell will degranulate and release a mixture of inflammatory mediators and potent chemoattractants for eosinophils into the surrounding tissues.458 These chemoattractants include histamine and small C-C chemokine proteins, including eotaxins (CCL11, CCL24, and CCL26) and CCL5, which appear to be particularly important in the selective recruitment of eosinophils. Other factors such as PAF and leukotriene B4 also function as chemoattractants, but these factors are not specific for eosinophils. TH2 lymphocytes and activated mast cells produce factors (IL-5, IL-3, and GM-CSF) that not only stimulate the production and release of eosinophils but also activate eosinophils and promote their survival.458
Helminths stimulate both humoral and cellular immunity, but they are resistant to killing by conventional immune mechanisms. B lymphocytes produce IgG antibodies that may bind to the parasites, but their extracellular cuticles cannot be penetrated by the complement membrane attack complex or T lymphocyte perforins. As previously discussed for allergens, specific IgE antibodies against parasite antigens are also produced, and these IgE molecules bind to mast cells. The binding of parasite antigens to these IgE antibodies results in mast cell activation and degranulation and release of inflammatory mediators and potent chemoattractants for eosinophils.458
Like molecules associated with neutrophil adhesive processes, selectins and β2 integrins are involved in eosinophil adhesion to activated endothelial cells. In addition, endothelial cells activated by IL-4 and IL-13 express vascular cell adhesion molecule-1 (VCAM-1), which binds to very late antigen-4 (VLA-4) on the surfaces of eosinophils. This integrin is not expressed on neutrophils and presumably helps to provide specificity for eosinophil localization.478
Eosinophils migrate into the tissues in response to chemoattractants generated in response to helminths. Initially, migration is stimulated primarily by mast cell- and/or parasite-derived attractants, including chitin (N-acetyl-beta-D-glucosamine), a widespread polymer that provides structural rigidity to helminths, insects, crustaceans, and fungi.393 A second wave of migration is supported by IL-5 and other cytokines produced by TH2 lymphocytes.458
Eosinophils bind to the opsonized parasites via their surface receptors to IgG and complement. The parasites are much too large for eosinophils to ingest, but when activated, eosinophils exhibit dramatic NADPH oxidase activity, which generates extracellular oxidants. They also exocytose their granules in the area of the invading parasite. Eosinophil peroxidase released from granules interacts with hydrogen peroxide generated from the respiratory burst and halide ions. This complex—along with other oxygen metabolites, major basic protein, eosinophil cationic protein, and eosinophil neurotoxin released from secondary granules—is primarily involved in the killing of helminths.458,478
The eosinophil has been perceived as a terminal effector cell in the TH2 inflammatory response. However, recent work indicates that eosinophils have the ability to modulate T lymphocyte responses. Eosinophils can present antigens to T lymphocytes and produce cytokines (primarily IL-4 and IL-13) that induce TH2-cell development and recruit additional TH2 cells to sites of inflammation.438
Basophil Functions
Basophils generally occur in low numbers in the circulation. They contain most of the histamine measured in blood. Histamine in granules is bound to proteoglycans (such as chondroitin sulfate and heparin), which are responsible for the metachromatic staining (purple color with blue dyes) of the granules. Basophils have biochemical characteristics similar to those of mast cells and share a common progenitor cell with mast cells in bone marrow, but they are clearly different cell types.164 Basophils have segmented nuclei and mast cells have round nuclei. Mast cells usually have more cytoplasmic granules than basophils. In cats, both primary and secondary granules in basophils are morphologically different from mast cell granules.
Basophils have functions similar to those of mast cells, including being important in the protective immunity against helminths.361 In contrast to mast cells, which develop and reside in tissues, basophils are recruited from blood into sites of inflammation after exposure to allergens, helminths, and ectoparasites.476 Among chemoattractants, chemokines that bind to C-C chemokine receptor type 3 (CCR3) are the most potent basophil chemoattractants. These chemokines include the eotaxins (CCL11, CCL24, and CCL26) and CCL5, CCL7, and CCL13.470 IL-33 has recently been identified as an interleukin that targets basophils. It enhances histamine release by IgE-dependent stimuli as well as the secretion of IL-4, IL-8, and IL-13 by blood basophils. IL-33 may also help regulate basophil binding to endothelium and migration into the tissues.470
Following the binding of an antigen to a specific, surface-bound IgE antibody, basophils are activated and release histamine and other mediators that contribute to the inflammation present in immediate hypersensitivity reactions. In addition to IgE-mediated antigenic activation, other substances including C5a, various bacterial peptides, and chemokines can also activate basophils.470 Recent studies reveal that basophils perform essential nonredundant functions in TH2 cytokine-dependent immunity. In particular, basophils migrate to lymph nodes and function as antigen-presenting cells, which appears to be critical for the induction of TH2-cell differentiation.411,431 In concert with IL-3, stem cell factor (SCF) prolongs basophil survival by delaying apoptosis.208
Monocyte/Macrophage/Dendritic-Cell Functions
Monocyte Functions
Monocytes are present in mammals, birds, amphibians, and fish.27 Several subsets of monocytes are reported to occur in mice and humans.27 One subset of monocytes appears to crawl along the luminal endothelium of blood vessels during the steady state, patrolling the endothelium independent of the direction of blood flow.27 Monocytes and their progeny have at least three major functions in mammals: phagocytosis, antigen presentation to T lymphocytes, and immunomodulation associated with the production of an array of cytokines involved in the regulation of inflammation and hematopoiesis. Monocytes may circulate in blood and return to the bone marrow under steady-state conditions or they may migrate into tissues, where they can differentiate into macrophages and dendritic cells. They are rapidly mobilized from the bone marrow in response to inflammatory conditions.506 Chemokines, including CCL2 and CCL7, promote this egress from bone marrow.27 Monocytes migrate in tissues in response to a similar array of chemoattractants to which neutrophils respond, including various chemokines, C5a, leukotriene B4, PAF, and bacterial products.106,160
Macrophage Functions
Macrophage colony-stimulating factor (M-CSF) stimulates not only monocyte production but also the transformation of monocytes into macrophages.122 M1 macrophages are activated by lipopolysaccharides (LPS) and interferon-γ (IFN-γ). They have potent antimicrobial properties, promote inflammation, and secrete IL-12, which stimulates T-helper 1 (TH1) responses. M2 macrophages appear to be more important in wound repair, tissue remodeling, and immunomodulation by stimulating TH2 responses.27,170
The development of monocytes into macrophages is associated with a marked increase in size, an increase in granules (lysosomes), an increase in the size and number of mitochondria, and an increase in phagocytic capacity. Macrophage function is augmented by various cytokines, the most potent of which is reported to be IFN-γ. M-CSF and GM-CSF also enhance macrophage function. The mononuclear phagocyte system consists of various macrophage subsets, including Kupffer cells in the liver, littoral cells in spleen, nurse cells in marrow, peritoneal and pleural macrophages, alveolar macrophages, and multinucleated giant cells that may form during chronic inflammatory conditions from a fusion of mononuclear phagocytes.160
Macrophages move more slowly and are generally less potent in killing bacteria, but they are notably more active against viral, fungal, protozoal, and helminth infections than are neutrophils. Macrophages can synthesize new membrane material and replace expended lysosomes. Therefore they have more staying power in combating infections than do neutrophils, which have limited synthetic abilities.106,160
Macrophages play important roles in linking the innate and adaptive immune responses. As part of the innate immune response, they express an array of microbial pattern-recognition receptors, including Toll-like receptors, which allow them to recognize a variety of bacterial molecules such as LPS from Gram-negative bacilli. Macrophages also have Fc receptors for antibodies and complement receptors, which promote their phagocytosis of opsonized microorganisms.160
The antimicrobial properties of macrophages are less well understood than those of neutrophils. NADPH oxidase and myeloperoxidase activities are present, although the activity of myeloperoxidase is considerably less than it is in neutrophils. Lysozyme is present, but few organisms are sensitive to it in their native states. Nitric oxide, a free radical generated from l-arginine, generally appears to be important in microbial killing by macrophages, especially after nitric oxide interacts with superoxide to generate toxic derivatives, including peroxynitrite.108,160,341
Macrophage scavenger receptors recognize not only microorganisms but also lipids and dying or dead cells.27 Macrophages demonstrate necrotaxis and necrophagocytosis (phagocytosis of devitalized tissue). Opsonization of necrotic tissue is not required for necrophagocytosis to occur. Consequently macrophages serve an important function in cleaning up necrotic tissue and other debris within the body.160
Macrophages have important functions in the modulation of immunity, including antigen processing; killing of tumor cells after sensitization by T lymphocytes; and synthesis of CSFs, interleukins, complement components, IFN, and TNF-α. Macrophages also remove aged or damaged erythrocytes. Most of the iron released from these phagocytized erythrocytes is rapidly returned back into the circulation, but some of it is stored in macrophages in the form of ferritin and hemosiderin. Macrophages are also necessary for normal wound healing.160 Macrophages in the liver and spleen are most important in clearing blood-borne pathogens of dogs, rodents, rabbits, monkeys, and humans, but intravenous pulmonary macrophages are most important in defending against blood-borne pathogens in cats, ruminants, pigs, and horses.62,430
Dendritic-Cell Functions
Dendritic cells develop from immature dendritic cells that leave the bone marrow, enter blood, and migrate into the tissues. Immature dendritic cells are estimated to account for about 5% of the monocyte-like cells in the blood of humans.27,506 Immature dendritic cells are capable of recognizing invading pathogens because they have pattern-recognition receptors (PRRs), including Toll-like receptors and nucleotide-binding oligomerization domain (NOD) receptors, on their surfaces, which bind to pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharides and microbial nucleic acids. Once immature dendritic cells bind and phagocytize an exogenous antigen, they rapidly mature and develop enhanced antigen-processing abilities. Engulfed antigens are partially digested when phagosomes containing antigens fuse with lysosomes. Resultant endosomes containing peptide fragments fuse with other endosomes containing newly formed MHC-II molecules. The MHC-II molecules combine with peptide antigens to form MHC-peptide complexes, which are presented on the cell surface for binding to CD4+ T lymphocytes with complementary T cell receptors (TCRs).
A subset of dendritic cells can process endogenous antigen and express both MHC-I and MHC-II on their surfaces. The antigen bound to MHC-I may be acquired if the dendritic cells are infected with a pathogen, or they may acquire the antigen from dying cells. Dendritic cells with foreign antigen bound to MHC-I and MHC-II molecules on their surfaces migrate to lymph nodes and present antigen to naive CD8+ and CD4+ lymphocytes, respectively.458
Plasmacytoid dendritic cells are produced in the bone marrow and migrate to the tissues. Although they can also process antigens and control T lymphocyte responses, they are specialized cells that respond to viral infections with a massive production of IFN-α.170
Monocytes can develop into inflammatory dendritic cells or TNF-α- and iNOS-producing (TiP) dendritic cells under inflammatory conditions. The main function of monocyte-derived inflammatory dendritic cells may be to kill organisms rather than to process antigens and regulate T lymphocyte function.27
Lymphocyte and NK Cell Functions
Lymphocytes are divided into T lymphocytes, B lymphocytes, and NK cells. A thorough discussion of the functions of these cells is beyond the scope of this text; the reader is therefore referred to immunology textbooks such as the one by Tizard458 for more detailed information.
T lymphocytes are largely responsible for cellular immunity. They are involved in immune regulation, cytotoxicity, delayed-type hypersensitivity, and graft-versus-host reactions. T lymphocytes are also actively involved in the control of hematopoiesis. To produce these effects, different subpopulations produce a large number of cytokines with diverse biological activities. Most T lymphocytes express TCRs composed of α and β chains. These TCRs recognize peptide antigens bound to MHC-I or MHC-II molecules. Peptides bound to MHC-I molecules are synthesized intracellularly by most cell types in the body, while peptides bound to MHC-II molecules are formed from extracellular antigens that have been endocytosed and processed by professional antigen-presenting cells, with dendritic cells being most important. Surface proteins CD8 and CD4 are coreceptors that bind to MHC-I and MHC-II, respectively.458
CD4+ T Lymphocyte Functions
Naive CD4+ T lymphocytes develop into subsets of T-helper (TH1, TH2, and TH17) lymphocytes through maturational processes induced by binding of processed antigens (bound to MHC-II on the surface of antigen-presenting cells) to complementary TCRs on the surface of the lymphocytes. Different sets of cytokines promote lineage differentiation.514 The binding of an antigen to its complementary TCR sends a signal through CD3 proteins that triggers a clonal proliferation of cells and the production of short-lived effector cells and long-lived memory cells. When these antigen-specific memory cells recognize the same antigen at a later date, they undergo a secondary heightened proliferative response. Dendritic cells are particularly important as antigen-presenting cells and appear to be required to activate naive T lymphocytes. Macrophages and B lymphocytes can also function as antigen-presenting cells with MHC-II on their surfaces.458
TH lymphocytes exert their control in defending against pathogens and neoplasia by recruiting and activating other immune cells, including B lymphocytes, CD8+ T lymphocytes, macrophages, mast cells, neutrophils, eosinophils, and basophils.514 TH1 lymphocytes produce IFN-γ, TNF-β, IL-2, and IL-3; support cellular immunity; and are particularly important in the defense against intracellular pathogens. TH2 lymphocytes produce IL-4, IL-5, IL-6, IL-10, IL-13, and IL-25; they also support humoral immunity and are essential in the defense against helminths and other extracellular pathogens. TH17 lymphocytes secrete IL-17, IL-21, and IL-22; generate strong proinflammatory effects; and are involved in fighting gram-negative bacteria, fungi, and some protozoa.144 Considerable heterogeneity and variable plasticity exist between TH lymphocytes.514
CD4+ T lymphocytes can also differentiate into regulatory T (Treg) lymphocytes. These Treg lymphocytes synthesize IL-10 and appear to suppress potentially deleterious effects of TH cytokine production. CD8+ Treg lymphocytes have also been identified but are less well studied. Altered T lymphocyte function may result in the development of autoimmune and allergic inflammation; Treg lymphocytes may help prevent the development of these disorders.96,172,231
CD8+ T Lymphocyte Functions
Dendritic cells with foreign antigen bound to MHC-I migrate to lymph nodes and present the antigen to naive CD8+ lymphocytes. The activation of naive CD8+ lymphocytes also requires IL-12 from activated dendritic cells and IL-2 and IFN-γ from TH1 lymphocytes that recognize the same antigen. Stimulated naive and memory CD8+ lymphocytes proliferate and develop into short-lived cytotoxic T lymphocytes and long-lived memory cells. When these antigen-specific memory cells recognize the same antigen at a later date, they undergo a secondary heightened proliferative response.458
Immunologic synapses form when TCR-CD8 complexes on the surface of cytotoxic T lymphocytes bind to target cells expressing complementary peptide antigens bound to MHC-I molecules on the surfaces of target cells. Following adhesion, cytotoxic proteins (including perforin, granzymes, and granulysin) within secretory lysosomes of cytotoxic T lymphocytes are exocytosed. Released perforin creates transmembrane channels that facilitate the entry of granzymes and granulysin into the cytoplasm of target cells, and these molecules induce apoptosis. Cytotoxic T lymphocytes also mediate apoptosis of target cells by binding between CD95L (Fas-ligand) expressed on their surfaces to CD95 (Fas) on the surface of target cells. This death receptor pathway is important as a mechanism for removing excess or self-reactive T lymphocytes.458
B Lymphocyte Functions
Naive B lymphocytes are exposed to antigens in lymph nodes and spleen. These recirculating cells enter lymph nodes through HEVs in the paracortex, a region that contains resident dendritic cells, recently migrated dendritic cells that have collected antigen from peripheral tissues, and CD4+ TH lymphocytes needed for the maximal activation of B lymphocytes to produce antibody and to undergo class switching and affinity maturation. A separate TH lineage termed follicular helper T lymphocytes (TFH) has been reported to promote B lymphocyte activation,282 but some authors have suggested that TFH cells may be a different state of one or more of the other TH lineages.514 These activated B lymphocytes may develop into extrafollicular plasma cells which mount early antibody responses to antigen, or they may migrate to follicles and promote the formation of germinal centers, which generate plasma cells that can secrete high-affinity antibody and memory B lymphocytes, which provide long-lasting protection against a repeated challenge with the same antigen.40
Most B lymphocytes in lymph nodes are located in follicles; consequently antigens in afferent lymph that enters the subcapsular sinus must gain access to follicular B lymphocytes. For small antigens, such as low-molecular-weight toxins, this may occur by diffusion; but dendritic cells, macrophages, or B lymphocytes appear to be required to carry large antigens (particulates, immune complexes, viruses, and bacteria) to the follicles. Resident follicular dendritic cells mediate the retention of antigens and function as potent accessory cells during B lymphocyte activation.40
Although B lymphocytes can interact with antigens in various forms, it appears that membrane-bound antigens are the predominant forms that initiate B lymphocyte activation. In contrast to the need to process protein antigens to small peptides for binding to TCRs on T lymphocytes, intact antigens are recognized and bound by B cell receptors (BCRs) on B lymphocytes. It is unclear whether antigens are internalized into nondegradative intracellular compartments and then recycled to the surfaces of macrophages, dendritic cells, and B lymphocytes or simply retained as antigen on the surface of these antigen-presenting cells. Macrophages and dendritic cells may bind antigens to a variety of surface receptors, including Fc receptors, complement receptors, pattern-recognition receptors, and/or lectin receptors that could present unprocessed antigen to B lymphocytes, and B lymphocytes are reported to bind antigen to complement receptors, thereby transporting antigen independently of their BCRs.40
B lymphocyte activation is initiated following the binding of antigens to their cognate BCRs. Not only does antigen binding initiate their activation, but B lymphocytes can process the bound antigen and present it along with MHC-II to specific CD4+ TH lymphocytes, which secrete cytokines that stimulate B lymphocyte proliferation and differentiation. IL-21, produced by TH lymphocytes (especially TFH lymphocytes), is a potent cytokine for the activation and proliferation of human B lymphocytes, for differentiation of these cells into plasma cells, and for stimulating antibody production by plasma cells. This interaction between B and T lymphocytes results in the simultaneous stimulation of humoral and cellular immunity.40,282,410 B cell activating factor (BAFF) also induces B lymphocyte proliferation and differentiation into plasma cells. This glycoprotein member of the TNF family is secreted by activated innate immune cells and appears to be an important factor in B lymphocyte homeostasis.102
Following activation, B lymphocytes are transformed into immunoglobulin-producing immunoblasts and subsequently plasma cells. IgM is initially produced by these cells, but with continued antigenic stimulation, IgG becomes the predominant antibody type produced. Clonal amplification of these cells results in the production of greater amounts of antibody against the foreign antigen. In addition to immunoglobulins, B lymphocytes produce cytokines that may influence the proliferation and/or function of other blood cell types.40,458
B lymphocytes provide a link between innate and adaptive immunity because B lymphocytes express Toll-like receptors in addition to antigen-specific BCRs. Antigen binding to Toll-like receptors on memory B lymphocytes may result in their activation and differentiation into immunoglobulin-secreting plasma cells independent of T lymphocytes.102
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree