CHAPTER 135 Estrous Synchronization and Artificial Insemination in Domesticated Cervids
A powerful and well-respected tool for disseminating the genetics of select sires in cattle that have superior traits of economic importance is artificial insemination (AI). It is believed that this same AI technique has the potential to rapidly improve genetic gain within the cervid industry. One of the primary requirements for the success of AI with both species, however, is the identification of elite sires. In the deer industry, this is currently a limiting factor. Phenotypic characteristics such as antler size and body weight are generally reviewed and measured in assessing elite sires with the goal of improving the herd genetic makeup. The reality is that these same characteristics are governed not only by genetic makeup, but also by management techniques and nutritional condition. Standards that have been determined and tracked in cattle that reflect performance recording and analysis must likewise be developed for the cervid industry for both males and females if AI is to reach its full potential with this species.
In the mid-1970s, Polish scientists reported the first successful use of surgical AI of red deer.1 In this trial, three calves were produced from 12 hinds. Likewise, successful nonsurgical AI with wapiti (Cervus elaphus canadensis) was reported in 1984.2 In 1984, Haigh3 inseminated two white-tailed deer (Odocoileus virginianus) with fresh-cooled semen utilizing the techniques of placing a bovine insemination pipette through a disposable vaginal speculum and depositing the semen at the os cervix. The result of this procedure was that both females delivered two live fawns. Likewise, successful surgical AI has also been reported in reindeer (Rangifer tarandus).4 Today, there are reliable transcervical techniques that minimize the stress on the donors and make the AI procedures more accessible and available to everyone at a reasonable cost.
ESTROUS SYNCHRONIZATION
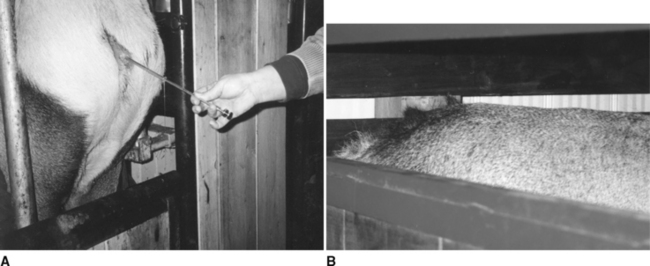
The success of any AI program is to breed the female at the most ideal time for conception. In cervids, this ideal is between 18 and 36 hours after the beginning of a standing heat. The challenge in achieving this is due to the difficulties faced in trying to observe the heat signs in the female. These signs are very subtle and often occur away from observers. Unfortunately, the most successful method of heat detection (the female being mounted by the male) is impractical in a field situation. A vasectomized male that has been marked with paint or oil both under the brisket and inside his front leg may be placed with the females. However, nobody goes through this trouble. There are two signs that are most certainly difficult to determine in a field situation, but should be evaluated at the time of AI. These signs are vaginal discharge of mucus and hair rubbed off the rump (Fig. 135-1). These signs do not have to be present in each female but it does help in assessing if the overall group of females is in good standing heat.
Because of the limitations of accurately identifying standing heat in the females, it is now an accepted standard practice to program the females with a progesterone device and perform time breeding after the removal of the device (Table 135-1). The CIDR (controlled intravaginal drug release) is the most commonly used progesterone-releasing device in the deer industry. Generally, when synchronizing wapiti or red deer crosses, the CIDR-b (1.9 g progesterone) is utilized, whereas the CIDR-g (0.33 g progesterone) is being used for smaller deer. Dependent upon the species, the device is left in between 10 and 14 days and then removed manually. Once the CIDR device is withdrawn, the blood progesterone levels drop, causing a luteinizing hormone (LH) surge and ovulation. Equine chorionic gonadotropin (eCG) or pregnant mare serum gonadotropin (PMSG) is often used in varying doses related to the different species, ensuring that synchronizing is tight. eCG tightens up the window of estrus by reducing the mean interval to the onset of estrus5,6 and may reduce the spread of ovulation.5
ARTIFICIAL INSEMINATION PROCEDURES
Traditional transcervical insemination techniques have been utilized in the breeding of wapiti and red deer hybrids since the 1980s, and just in the last few years for red deer. Purebred hinds can be transcervically inseminated with purebred wapiti semen, and this approach can be very effective in producing hybrids.7 To accomplish AI with these species, the use of a chute system or squeeze to restrain the female is required. This minimizes, or eliminates, the need for any tranquilization. The goal is to introduce the semen directly into the body of the uterus, just in front of the cervix. With the unusual problem females, the semen could be placed intracervically, and a pregnancy rate of 20% to 50% (depending on the semen quality) can still be achieved. Last year on 1491 transcervically inseminated wapiti females, an overall calving rate of 71.8% was achieved, with a 4.7% twinning rate. A similar calving rate of 73.5% on 950 red deer hinds was obtained, with a twinning rate of 4.1%.
Smaller deer are primarily inseminated laparoscopically. Laparoscopic AI is done with the tranquilized hind secured to a “stretcher table.” The pelvic area is lifted to a 40- to 60-degree angle, and through the laparoscope, the semen is deposited at the tip of the horn, ipsilateral to the preovulatory follicle, or half the dose of semen being deposited at the tip of both horns if ovaries are not visualized. The procedure requires only minutes to do, after which the tranquilizer is reversed and the female immediately walks away. This laparoscopic technique is a less invasive technique than the traditional laparotomy; however, the technician’s skills play a major role in the success of this technique. There are slight possibilities of adhesions and scar tissue formation, which might interfere with future conception or complicate a pregnancy. The personnel required and the cost of AI is increased with this technique, and the success rate is expected to be around 70%.
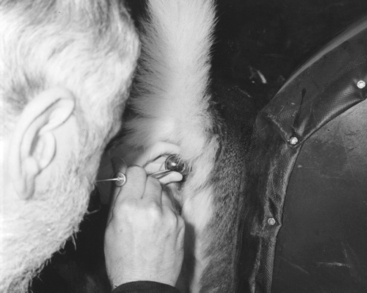
Recent breeding results in white-tailed does, utilizing nonsurgical techniques, are also encouraging. White-tailed does appear to be highly fertile and some females will become pregnant if the semen is deposited at the os cervix. However, better results will be obtained if cervical intraluminal semen deposition is achieved over a short space of time. The cervix is visualized through a vaginal speculum and a bovine embryo transfer pipette is fed through the cervix, into the uterus if possible (Fig. 135-2). White-tailed does are inseminated with this technique with or without synchronization. If no synchronization is used, a teaser buck is mixed with the females. When the doe “stands,” she is then inseminated. Results have been quoted as high as 80%, although they are quite variable. Last year, 35 does were inseminated 62 to 63 hours post-CIDR removal. Twenty-four females delivered at least one fawn (68.5% fawning rate), of which two carried twins, three carried triplets, and one carried quadruplets (but all died as stillborn) to term. This was the only farm that confirmed the parentage based on DNA testing. Another group has been inseminating the does at 50 to 52 hours after CIDR removal with an injection of 200 IU of eCG.8 Over the last 2 years, this group has inseminated over 1800 does, with variable results, being from almost 0% to almost 100% fawning rate, with an overall mean of 65%. This program produces about 3% multiple births with 5 fawns and average of 2.8 fawns per doe.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree