CHAPTER 17 Equine Ophthalmology
EXAMINATION
The equine eye presents particular challenges for diagnostic and therapeutic approaches. However, the basic principles of a complete ophthalmic examination hold true. Box 17-1 lists the equipment required for a routine ophthalmic examination. One should perform the initial ophthalmic examination in a well-lighted environment and before tranquilization. The examiner assesses facial, orbital, and eyelid symmetry; looks for ocular discharge (serous, mucoid, mucopurulent) or blepharospasm; and evaluates the cranial nerves, specifically cranial nerves II through VII. A complete ophthalmic examination includes assessment of pupillary light and menace response, maze testing, globe position and mobility, sensation of ocular and adnexal structures, and eyelid position and function. To evaluate direct and consensual pupillary light responses accurately often requires a bright focal light source (3.5-V halogen) and a darkened examination area.
BOX 17-1 EQUIPMENT FOR BASIC EQUINE OPHTHALMIC EXAMINATION
Modified from Wilkie DA: Ophthalmic procedures and surgery in the standing horse, Vet Clin North Am Equine Pract 7:535–547, 1991.
Further examination or therapy may require some form of tranquilization, regional nerve blocks, and topical anesthesia. Detomidine (Dormosedan, Pfizer Animal Health, Exton, Pa.) administered intravenously at 0.01 to 0.02 mg/kg or xylazine (Rompun, Haver-Lockhart, Shawnee, KS) at 0.5 to 1.0 mg/kg combined with butorphanol tartrate (Torbugesic, Bristol Laboratories, Inc., Evansville, Ind.) at 0.01 mg/kg administered intravenously provide a synergistic analgesic effect and facilitate examination, sample collection, intraocular pressure determination, nasolacrimal irrigation, lavage tube placement, and minor surgical procedures. If one intends to determine intraocular pressure (IOP), it is important to note that intravenous administration of xylazine significantly lowers the IOP.1
Sensory innervation of the globe and adnexa is from the trigeminal nerve (cranial nerve V), and motor innervation is from the facial nerve (cranial nerve VII). The ophthalmic nerves blocked most frequently are the auriculopalpebral branch of cranial nerve VII and the supraorbital (frontal) branch of cranial nerve V. Blocking of these nerves provides akinesia and anesthesia of the superior eyelid, respectively. One can palpate the auriculopalpebral nerve as it courses over the zygomatic arch in the area of the temporofrontal suture. Using a 25-gauge, 5/8-inch needle, one blocks the nerve by injecting 3 to 5 ml of mepivacaine (Carbocaine, Winthrop Laboratories, New York, N.Y.) over the zygomatic arch in this area. One blocks the supraorbital nerve as it emerges from the supraorbital foramen of the frontal bone by palpating the foramen, inserting a 25-gauge, 5/8-inch needle into the foramen, and injecting 2.0 ml of mepivacaine; one infuses another 1 to 2 ml subcutaneously while removing the needle. Additional sensory nerves that are occasionally blocked include the infratrochlear, lacrimal, and zygomatic branches of cranial nerve V.2 Alternatively, local infiltration of anesthetic can be used to provide anesthesia of a specific area. Finally, a retrobulbar nerve block may be performed for standing surgical procedures or to assist with general anesthesia. A retrobulbar block is performed using an 8-mm spinal needle. The needle is placed posterior to the dorsal bony orbital arch and directed ventrally and posterior to the globe. When the needle comes into contact with the dorsal rectus muscle of the eye, the globe will be seen to deviate dorsally. The needle is advanced just past the dorsal rectus muscle into the orbital muscle cone, and 5 to 8 ml of carbocaine is injected.
A complete ophthalmic examination can now be performed (see Box 17-1). The practitioner should obtain samples for bacterial or fungal culture or evaluation of tear production before instilling any topical solution or ointment on the eye. Aqueous production of tears is measured by using commercially available Schirmer tear test strips, with normal wetting being 20 mm or greater in 30 seconds. For a complete ophthalmic examination, the pupil should be dilated. This is performed using 1% tropicamide (Mydriacyl® Alcon Laboratories, Fort Worth, Texas) and will take 15 to 20 minutes for adequate dilation. Failure to dilate the pupil will result in abnormalities of the lens and posterior segment being overlooked. Using a bright focal light source, the practitioner now examines the conjunctiva, nictitans, cornea, anterior chamber, iris, pupil, and lens. The practitioner evaluates the ocular media (cornea, aqueous humor, lens, and vitreous) for clarity and transparency; assesses the position and size of the lens, shape and mobility of the pupil, and appearance of the corpora nigra; and examines the eye for irregularities, vascularization, and pigmentation. Although it is possible to examine the whole anterior segment using a Finoff transilluminator with or without magnification, a new handheld monocular slit lamp that attaches to an otoscope or ophthalmoscope handle is available (HSL-10, Heine, United States). The handheld slit lamp provides magnification and an appreciation for depth and three-dimensional anatomy from the anterior vitreous forward. Fluorescein staining of the cornea detects the presence of corneal ulceration, and the appearance of fluorescein at the nares indicates a patent nasolacrimal system. Some ophthalmologists also use rose bengal to stain the cornea. Rose bengal will stain damaged or devitalized corneal epithelial cells and may be more sensitive than flourescein for early fungal or herpetic disease. After instillation of topical anesthesia (proparacaine 0.5%, Alcaine, Alcon Laboratories, Fort Worth, Texas), the clinician can cannulate the nasolacrimal puncta using a 3.5F urinary catheter and irrigate the nasolacrimal system. Alternatively, one can irrigate the nasolacrimal system in retrograde fashion, cannulating the duct using a 5F catheter at the nasal opening. Using thumb forceps, one can grasp the nictitans and examine the palpebral and bulbar surfaces for foreign bodies, mass lesions, or other abnormalities. Conjunctival and corneal cells are collected for cytologic study using a Kimura spatula after administration of topical anesthesia. If available, Tonopen Vet (Reichert Ophthalmic Depew, N.Y.) can be used to determine ocular pressure.
OCULAR ULTRASONOGRAPHY
Indications
Ocular ultrasound is indicated for evaluation of intraocular contents when one or more of the ocular transmitting media are opaque, including opacification of the cornea, aqueous and vitreous humors, and the lens. The most common indications for ocular ultrasound are in eyes with a cataract to evaluate for a retinal detachment, after traumatic hyphema to assess posterior segment damage, or in eyes with severe corneal opacification. In addition, ocular ultrasound can be used to evaluate the orbit in instances of exophthalmos or orbital trauma. UBM can be used to determine depth of a corneal lesion such as a squamous cell carcinoma or to examine an anterior uveal mass for extent of involvement.
EYELIDS
Congenital
Diseases of the equine eyelid are common. Congenital lesions include coloboma, agenesis, dermoid, and entropion.3,4 Of these, entropion is the only frequently occurring disease. Entropion is inward rolling of the eyelid margin that results in facial hairs coming into contact with the cornea, leading to irritation, conjunctivitis, and possibly secondary corneal ulceration. Causes include prematurity or dehydration in foals with enophthalmos, ocular pain resulting in spastic entropion, and primary conformational problems. Manual reduction and frequent topical ocular lubrication with artificial tear ointment is the initial treatment of choice and may be the only treatment required in some foals, especially if the entropion follows dehydration. If the entropion is severe or does not respond to manipulation, the clinician may place two or three temporary vertical everting mattress sutures to position the eyelid correctly.3 It is important to avoid overcorrection, which could lead to the inability to close the eyelids during blinking. Suture placement is at and perpendicular to the eyelid margin, tacking this to the periocular skin over the orbital rim. Monofilament, 3–0 to 5–0 nylon suture is preferrable; the sutures are removed after 10 to 14 days. The clinician must treat associated corneal disease to prevent secondary infection and subsequent scar formation. Entropion requiring more aggressive surgical intervention in the form of skin excision is rare. Severe or recurrent entropion is correctable by excising an elliptic portion of eyelid skin in the affected area and reapposing the skin edges (Hotz-Celsus procedure).
Acquired
Common acquired conditions involving the equine eyelid include trauma and neoplasia.
TRAUMA
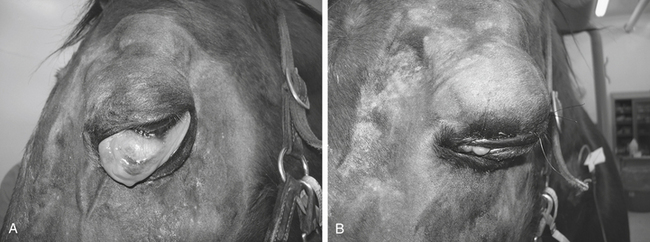
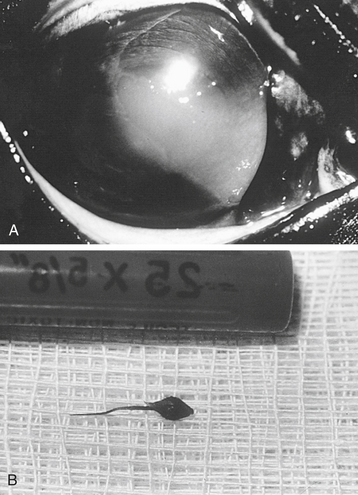
Eyelid trauma includes contusions and lacerations.5 Commonly associated with eyelid trauma are corneal abrasions or lacerations; anterior uveitis; and, if the trauma involves the medial canthus, nasolacrimal system damage. Eyelid contusions often result in blepharoedema and hemorrhage. Although mild blepharoedema does not require therapy, recovery can be hastened by using systemic flunixin meglumine (Banamine, Schering Corp., Kenilworth, New Jersey), cold compresses in the acute phase, and warm compresses beginning the day after the injury. In additon to the aformentioned therapy, severe blepharoedema may also require a temporary tarsorraphy and elevation of the head to protect the tissues and resolve the swelling (Figure 17-1).
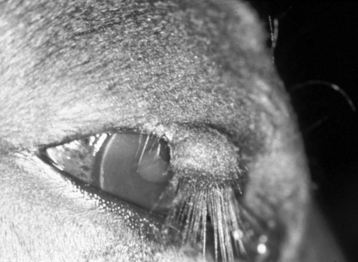
Eyelid lacerations are more serious and usually require immediate therapy (Figure 17-2). The vascular supply to the eyelid is extensive, and many apparent avascular segments of eyelid recover after repair. If possible, primary wound closure is preferable. It is important to remove all debris from the wound before closure, disinfect the eyelid surface and adjacent tissues with a 1:25 to 1:50 dilution of povidone-iodine solution, avoid excessive tissue débridement, and under no circumstances amputate a pedicle of eyelid. Loss of eyelid margin, whether iatrogenic or the result of trauma, leads to severe, chronic corneal irritation; vascularization; ulceration; and fibrosis (Figure 17-3). In addition, reconstructing an eyelid margin from the adjacent skin after amputation of the normal eyelid margin is difficult.
One should suture lacerated eyelids using a two-layer closure, ensuring accurate anatomic apposition of the wound edges and eyelid margin. Minor surgical repairs can be performed using sedation and local nerve blocks, but the horse may require general anesthesia if the injury is severe. First, suture the deeper, conjunctival layer using 5-0 to 6-0 absorbable suture in a horizontal mattress pattern beginning away from and working toward the eyelid margin, taking care to avoid penetrating the conjunctiva so that the suture does not come into contact with the cornea. The practitioner then closes the skin with 5–0 to 6–0 nonabsorbable monofilament suture. The eyelid margin is the most important part of wound closure and is closed first to ensure accurate apposition. The author prefers to use a cruciate suture pattern at the eyelid margin and a simple interrupted pattern for the remainder of the skin closure. Failure to perform a two-layer closure or achieve precise apposition of the eyelid margin may result in ulcerative keratitis or other secondary complications.6 Postoperative therapy includes administration of antibiotics for 5 to 7 days for systemic effect; tetanus toxoid; warm, moist compresses; and flunixin meglumine if inflammation and swelling are a problem. Topical medication is not required for eyelid injuries unless corneal or anterior segment damage accompanies the injury. The practitioner must evaluate eyelid function, and the eyelid must provide adequate protection to the cornea. If the blink response is impaired, the cornea should be protected with topical lubricants as often as possible. Advanced blepharoplastic techniques for repair of severe eyelid trauma with resulting loss of tissue are beyond the scope of this chapter but are discussed in detail elsewhere.3
NEOPLASIA
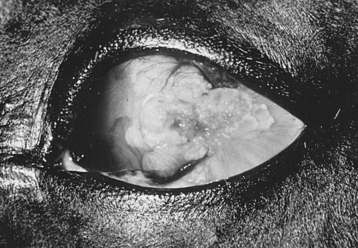
The most common neoplasms of the equine eyelid are sarcoid and squamous cell carcinoma. In addition, melanoma, mast cell tumor, lymphosarcoma, basal cell carcinoma, and papilloma can affect the eyelid (Figure 17-4). The differential diagnosis for eyelid neoplasms includes parasitic diseases such as ocular habronemiasis and other causes of granulomatous skin disease. Treatment of eyelid neoplasia depends on the location, size, tumor type, age and purpose of the horse, cost, surgical skill, and equipment available. Treatment modalities include surgical excision, radiation therapy, intralesional chemotherapy, hyperthermia, immunotherapy, cryosurgery, or a combination of these. The aim of therapy is to eliminate or halt progression of the tumor while maintaining eyelid function and preserving the eye and vision. Complete excision with primary closure is the optimal treatment but often cannot be achieved because of limitations on availability of tissue for reconstruction and the extensive and aggressive nature of many eyelid neoplasms. If the veterinarian cannot appose eyelid margins after tumor excision, more involved blepharoplastic techniques, such as advancement skin flaps, may be indicated (Figure 17-5).
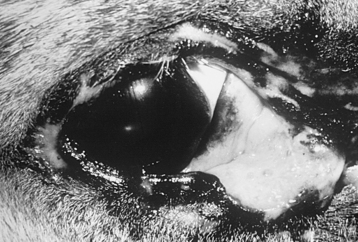
FIGURE 17-5 Squamous cell carcinoma involving the medial canthus and inferior eyelid of a 19-year-old Appaloosa.
Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) involving the eyelid is usually erosive and ulcerative.5 The medial canthus is the most common site of origin (see Figure 17-6). Invasion of adjacent soft and bony orbital tissue is possible if the disease is untreated. Treatment of ocular SCC is discussed in detail in the sections on conjunctiva and third eyelid.
Sarcoid
Periocular sarcoid is the second most common eyelid neoplasm of the horse. The average age of an affected horse is 4 years, and smooth and warty sarcoids occur. Orbital invasion and bony involvement are possible. Surgical excision is associated with frequent recurrence and often is combined with cryosurgery, hyperthermia, chemotherapy, or irradiation to ensure complete tumor destruction. Of all treatment modalities, radiation is the most expensive and yields the best overall success.7
Intralesional immunotherapy with cell wall extracts of bacillus Calmette-Guérin (BCG) in oil has been used effectively in repeated injections 3 weeks apart. A total of four doses usually is required for complete tumor regression, with the overall response reported to be 69%, provided the injection was intralesional.7 Injection of cell wall extracts of BCG is associated with local inflammation and tumor necrosis. During this period of inflammation, the clinician must protect the globe to prevent corneal desiccation or ulceration. The use of live BCG organisms or whole killed organisms has been associated with anaphylactic reaction and death and is discouraged. Adverse systemic reactions to cell wall extracts of BCG are rare, but premedication with systemic corticosteroids is advised.
Intralesional chemotherapy with cisplatin in an oil-water emulsion has been reported to result in regression of equine sarcoid.8 A series of four injections, spaced 2 weeks apart, with a mean dose of cisplatin of 0.97 mg/cm3 of tumor mass resulted in tumor regression in all treated horses. In addition, 87% of horses treated for sarcoids were relapse free at a 1-year follow-up. A larger study of intralesional cisplatin reported a cure rate of 96.3% for sarcoids and 88% for SCC at 4 years after treatment. 9
CONJUNCTIVA AND THIRD EYELID
The conjunctiva is divided into bulbar and palpebral surfaces. The conjunctiva merges with the cornea at the limbus, with the eyelid at the eyelid margin, and the bulbar and palpebral conjunctiva join to form the fornix. The conjunctiva has normal resident bacterial and fungal populations. The most common isolates from the equine conjunctiva vary according to geography, season of the year, and investigator.10–12 In general, isolates of gram positive organisms—Corynebacterium, Bacillus, Staphylococcus, and Streptomyces—are more common than gram negative organisms from normal equine conjunctiva.11 Fungi are isolated more commonly in the summer and autumn and have been reported to be present in 95% of normal horse conjunctiva samples.12 In addition, fungi are isolated more frequently from stabled horses, which likely relates to the immediate environmental and husbandry conditions.11
Unlike the small animal patient, horses rarely acquire infectious conjunctivitis. Organisms reported to result in infectious conjunctivitis in the horse include equine herpesvirus type 2,13 Mycoplasma spp., Chlamydia, Moraxella equi, and mycotic and viral agents.3
Foreign bodies and trauma are common causes of conjunctival irritation and damage and often are associated with concurrent eyelid and corneal injuries. The clinician directs treatment to eliminate the cause, treat any conjunctival or associated eyelid or corneal damage, and prevent secondary infection. A complete ophthalmic examination is necessary in all animals with evidence of ocular trauma. Conjunctival foreign bodies should be considered in any horse with a recurrent corneal erosion, ocular pain, or conjunctivitis. The clinician should examine the superior and inferior fornices and the bulbar and palpebral surface of the nictitans after topical administration of 0.5% proparacaine. Magnification is essential to detect foreign bodies. In addition, irrigation of the nasolacrimal system may yield foreign material associated with chronic conjunctivitis or dacryocystitis.
Severe chemosis with conjunctival prolapse and exposure may result from self-trauma, especially associated with being recumbent for a prolonged time. Treatment includes systemic anti-inflammatory drugs; artificial tear ointment to protect exposed conjunctiva; elevation of the head; and, in severe cases, repositioning of the conjunctiva and a temporary tarsorraphy until the swelling resolves (see Figure 17-1).
Parasites
Common parasites of the equine conjunctiva include Thelazia lacrymalis, Habronema spp., and Onchocerca cervicalis.14 In a necropsy sample the prevalence of Thelazia organisms in the conjunctival sac of horses is highest in young animals, with 43% of 1- to 4-year-old horses being affected.15 In most instances infection is not associated with clinical signs, but chronic conjunctivitis, seromucoid discharge, and conjunctival nodules can occur.3 The adult parasite may be visible on ophthalmic examination of the cornea and conjunctival fornix, and larvae may be visible on examination of centrifuged samples of nasolacrimal washes. Treatment includes removal of the adult worms, irrigation of the nasolacrimal system, topical corticosteroids to reduce inflammation, and fly control. The topical organophosphates echothiophate, 0.03% to 0.06% every 12 hours, or isoflurophate, 0.025% every 24 hours for 7 days, have been reported to kill the parasite.16 Systemic treatment with fenbendazole at 10 mg/kg orally every 24 hours for 5 days also is reported to be effective against Thelazia organisms.17
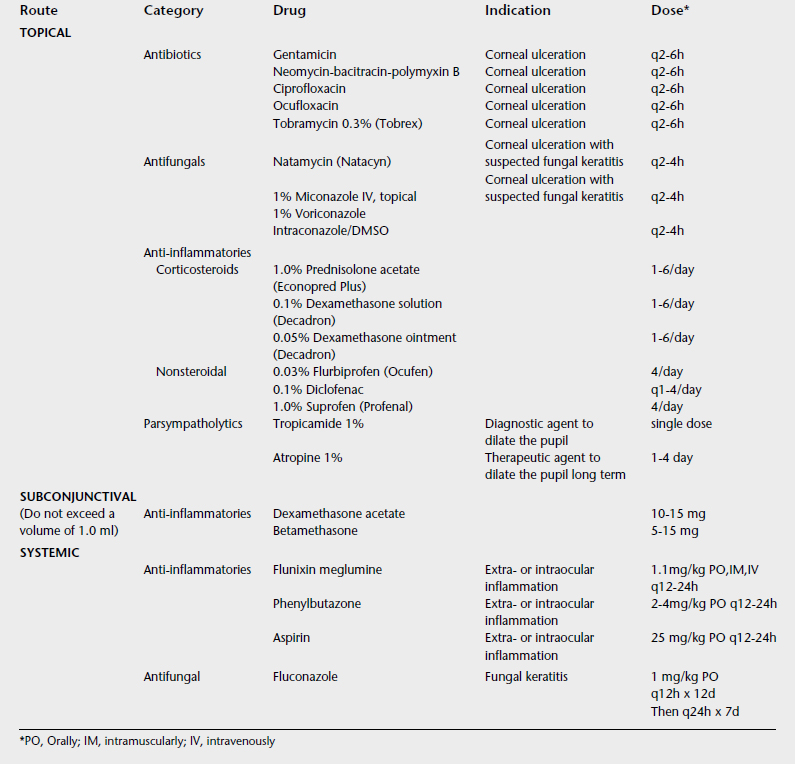
Ocular manifestations of equine cutaneous habronemiasis result when the house or stable fly deposits the larvae of the Habronema spp. on or around the eye.3,18,19 The resulting granuloma is associated with inflammation, intense pruritus, and epiphora. Yellow, caseous granules are notable throughout the granulation tissue. Histologically, eosinophils and mast cells predominate. Diagnosis is based on clinical signs, location, season of the year, and histologic findings. Clinical appearance can simulate squamous cell carcinoma. Histologically, the differential diagnosis is equine cutaneous mastocytosis.3,19 Ocular lesions are most common at the medial canthus and may involve the skin, nasolacrimal duct, third eyelid, or conjunctiva. Treatment of equine cutaneous habronemiasis varies, and no single treatment is routinely successful.18 Surgical excision, cryosurgery, local and systemic corticosteroid therapy, systemic ivermectin (single dose, 0.2 mg/kg intramuscularly), various larvicidal treatments in the form of topical and systemic organophosphates, and various topical formulations containing ronnel or metrifonate with a dimethyl sulfoxide vehicle are used3,18,19 (Table 17-1). Dimethyl sulfoxide serves as a vehicle and has anti-inflammatory effects, possibly related to its ability to detoxify hydroxyl radicals generated by neutrophils. The aim of therapy is to kill the larvae while controlling the resulting inflammation. In addition, fly control is an essential part of an overall management program.
TABLE 17-1 Topical Preparations Used to Treat Equine Cutaneous Habronemiasis
| Ingredients | Amount |
|---|---|
| Ronnel solution (33%)13 | 60 ml |
| Thiabendazole (33%)13 | 120 g |
| 9-Fluoroprednisolone acetate13 | 60 mg |
| Dimethyl phthalate (24%)13 | 30 ml |
| Dimethyl sulfoxide13 | 500 ml |
| Nitrofurantoin ointment14 | 225 g |
| Ronnel solution14 | 20 ml |
| Dimethyl sulfoxide14 | 20 ml |
| Dexamethasone solution14 | 20 ml |
| Nitrofurazone ointment (0.2%)15 | 135 g |
| Trichlorfon (12.3%)15 | 30 ml |
| Dexamethasone (2 mg/ml) 15 | 30 ml |
| Dimethyl sulfoxide15 | 30 ml |
The blood-sucking midge Culicoides nubeculosus transmits O. cervicalis microfilariae. After transmission the immature microfilariae migrate through lymphatics and can travel to ocular and adnexal tissues. The prevalence of equine onchocerciasis increases with age, with an overall prevalence of 50% to 60%.14,20–22 The prevalence of onchocerciasis on histologic examination of the lateral bulbar conjunctiva is 10.8%. The presence of conjunctival microfilaria does not correlate with clinical abnormalities. Ocular onchocerciasis has been proposed as a cause of keratitis, conjunctivitis, equine recurrent uveitis, and depigmentation of the temporal bulbar conjunctiva.22 The pathogenesis is thought to involve the death of the microfilariae, release of antigens, and development of hypersensitivity in susceptible horses. Treatment is indicated in those horses with active conjunctivitis, keratitis, or uveitis attributable to onchocerciasis and includes anti-inflammatory agents such as topical corticosteroids and systemic flunixin meglumine; topical echothiophate iodide 0.025%; and systemic diethylcarbamazine, levamisole, or ivermectin3 (Table 17-2).
TABLE 17-2 Systemic Treatment of Ocular Onchocerciasis
| Drug | Dose |
|---|---|
| Diethylcarbamazine | 4.4-6.6 mg/kg/day orally for 21 days |
| Levamisole | 1.1 mg/kg/day orally for 7 days |
| Ivermectin | 0.2-0.5 mg/kg intramuscularly |
Neoplasia
Neoplasms affecting the conjunctiva and third eyelid include SCC, lymphosarcoma, melanocytoma, mastocytoma, hemangioma, and angiosarcoma.3,16 Diagnosis and characterization of adnexal neoplasms are based on history, signalment, location, appearance, and histopathologic examination. Routine surgical biopsy is performed with sedation, local nerve blocks, and topical anesthesia. Most adnexal neoplasms are benign or locally invasive, the exception being ocular angiosarcoma, which is reported to have a high incidence of metastasis.23 Treatment of adnexal neoplasms varies according to tumor type, location, and size; use of the animal; treatment modalities available; and cost. The aim of treatment is to eliminate the tumor, restore normal anatomy, and maintain function of the eye and associated structures.
SCC is the most common tumor of the equine eye and adnexa (Figure 17-6). Involvement can be unilateral or bilateral, with the third eyelid and medial canthus affected most frequently.16,24 Involvement of the corneal limbus, sclera, conjunctiva, and other adnexal structures also has been reported.16,24,25
The mean age of onset for SCC is 9.8 years for all horses compared with 3.8 years for ocular sarcoidosis.25 The incidence of ocular SCC is higher in draft-breed horses and Appaloosas than in light horses,26,27 and sexually intact males and females have a decreased incidence.26 Adnexal hypopigmentation and increased exposure to actinic radiation25,26 are hypothesized to predispose horses to develop ocular SCC.
Ocular SCC is malignant and locally invasive with the potential to metastasize.22,28 Metastases to local and cranial mediastinal lymph nodes, parotid salivary glands, and the thoracic cavity occur in 10% to 15% of patients.27
Treatment of ocular SCC varies and must be designed for the individual patient. Surgical excision,25 radiofrequency hyperthermia,29 cryotherapy,30 immunotherapy,30 radiation therapy,25,28–30 intralesional chemotherapy,8,31 laser surgery,5 or a combination of these29,30 have been used with success. Location, size, and depth of the tumor; visual status; financial commitment; purpose of the animal; and the presence or absence of metastatic disease influence the choice of therapy.
Most SCCs are radiosensitive and can be treated successfully with sources of β- or γ-radiation. The major disadvantage of less expensive modes of radiation therapy, such as strontium 90 (90Sr), a source of β-radiation, is the size limit of tumor amenable to treatment. With 90Sr, for example, half the β-radiation produced is lost after passage through 1 mm of soft tissue. Thus the practitioner should limit treatment to lesions less than 2 mm deep, such as corneal, scleral, or superficial conjunctival SCCs. Radiation is most appropriate as an adjunct therapy after surgical removal of a corneal or conjunctival SCC. When used appropriately, 90Sr can achieve a nonrecurrence rate for SCC of 87.5% after 2 years.32
The use of interstitial radiotherapy for periocular SCC has been reported.29,32 Radioactive gold seeds (198Au) were used to impart a medium-energy γ-ray (0.41 MeV) with a half-life of 93.3 hours for a total recommended dose of 5000 rad.29 Success rates of 80% at 1 year and 70% at 2 years are reported.32 Disadvantages of radioactive implants are their substantial cost, their limited availability, the risks associated with human exposure, and the licensing restrictions on their handling.29 Secondary complications of interstitial radiation therapy include temporary corneal opacity, local necrosis, hair loss, damage to normal structures, and local depigmentation.29,32 Although no retinal or lens changes have been reported after interstitial radiation treatment, the practitioner should consider the possibility of photoreceptor damage and cataractogenesis following radiation therapy.
Radiofrequency hyperthermia involves the passage of a radiofrequency (2 MHz) electric current between two electrodes. Tissue between these probes offers resistance, resulting in thermal energy being transferred to the tissue. Tissue temperature increases to 50° C in a 1-cm2 area,29 with malignant cells exhibiting a greater sensitivity to thermal energy than normal cells. The practitioner should not use topical radiofrequency hyperthermia as the only mode of treatment for tumors extending deeper than 3 mm or those greater than 4 to 5 cm in diameter. Superficial eyelid SCCs or corneal and conjunctival SCCs are most appropriate for treatment using radiofrequency hyperthermia. The practitioner should be careful not to overlap the fields of exposure, especially in corneal tumors, because this creates a risk of excessive necrosis of normal tissue.33
Intralesional chemotherapy with cisplatin or bleomycin in an oil-water emulsion has been reported to result in regression of equine SCC. Treatment is the same as previously described for eyelid sarcoidosis. Of those horses with adnexal SCC treated with intralesional cisplatin, 65% to 93% were relapse free at 1 year.8,31 A second study reported a success of 88% at 4 years.9
NASOLACRIMAL SYSTEM
The nasolacrimal system consists of the superior and inferior puncta and canaliculi, nasolacrimal sac, nasolacrimal duct, and nasal punctum located on the floor of the nasal vestibule.34 The nasolacrimal system serves to carry tears from the medial canthus of the eye to the nasal vestibule. Abnormalities of the nasolacrimal system result in epiphora, an overflow of tears, following an impairment in drainage of tears. Epiphora can be serous, mucoid, or mucopurulent and must be differentiated from reflex lacrimation resulting from ocular irritation or inflammation. Examination of the nasolacrimal system includes placement of fluorescein stain in the conjunctival cul-de-sac and observing its appearance at the nasal opening. The nasolacrimal system can be irrigated antegrade from the eyelid punctum or retrograde from the nasal punctum. The practitioner applies topical anesthetic (0.5% proparacaine) to the eye and places a 3.5F open-ended feline urinary catheter in the superior punctum. Sterile eyewash or saline (10 to 20 ml) is used to irrigate the system, and fluid is observed passing out of first the inferior punctum and subsequently the nasal punctum. If performing retrograde irrigation from the nasal punctum, the practitioner uses a 5F or 6F urinary catheter and increases the volume of irrigating solution. Radiographic imaging of the nasolacrimal duct, dacryocystorhinography, can be performed using contrast material injected into the duct from the superior eyelid punctum.3,34Lateral and oblique radiographic views are best, and useful contrast materials include 60% barium sulfate (Novapaque, Picker Corp., Cleveland, Ohio) and 31% diatrizoate meglumine and diatrizoate sodium (Renovist II, Bristol-Myers Squibb, Princeton, N.J.). Indications for dacryocystorhinography include chronic epiphora, inability to irrigate the nasolacrimal duct, suspicion of a nasolacrimal foreign body, and evaluation of the nasolacrimal duct for congenital or acquired abnormalities. The nasolacrimal duct can also be imaged using a computed tomography (CT) scanner.
Abnormalities of the nasolacrimal system can be congenital or acquired. In the horse the most common congenital abnormality is atresia of the distal portion of the nasolacrimal duct and the nasal punctum, which results in mucoid epiphora at 3 to 4 months of age.3,35Additional congenital abnormalities include atresia of an eyelid punctum, abnormal placement of an eyelid punctum, and multiple nasal openings.3,16 Whether these conditions are inherited is unknown. To correct an atretic nasal opening, the clinician passes a catheter from the inferior eyelid punctum to the level of the atresia. The clinician then palpates the end of the catheter in the nasal vestibule and makes an incision through the nasal mucosa overlying the catheter, exposing the catheter. The clinician then brings the tubing out the nasal opening and, along with the portion of tubing from the inferior punctum, sutures it to the skin of the face. The tubing is left in place for 3 to 4 weeks, and topical broad spectrum ophthalmic antibiotic solution is administered during this time.
CORNEA
Ulcerative Diseases
CLINICAL SIGNS AND DIAGNOSIS
A corneal ulcer is a break in the corneal epithelium. Clinically, ulceration results in lacrimation, blepharospasm, photophobia, conjunctival hyperemia, corneal edema, and possibly miosis and aqueous flare. The diagnosis of a corneal ulcer is based on these clinical signs and fluorescein staining of the cornea. The underlying stroma retains the fluorescein stain and appears green. Before instilling any therapeutic or diagnostic agents in the eye, the veterinarian should submit bacterial and fungal culture samples from all corneal ulcers in the horse and should obtain culture samples from the margin of the ulcer. After obtaining culture samples and staining the cornea with fluorescein, the veterinarian applies topical anesthetic and obtains a scraping from the ulcer for cytologic examination. The cells are placed on a glass slide and stained to allow examination for bacteria, fungal hyphae, and cell type. Gram and Giemsa stains work well for examination. The presence of gram negative rods indicates the possibility of an infection with Pseudomonas spp., whereas cocci are suggestive of Streptococcus spp., a frequent and severe corneal pathogen. The presence of fungal hyphae is pathognomonic for mycotic keratitis, with Aspergillus spp. and Fusarium spp. being the most frequent corneal pathogens. Mixed bacterial and fungal infections are common.
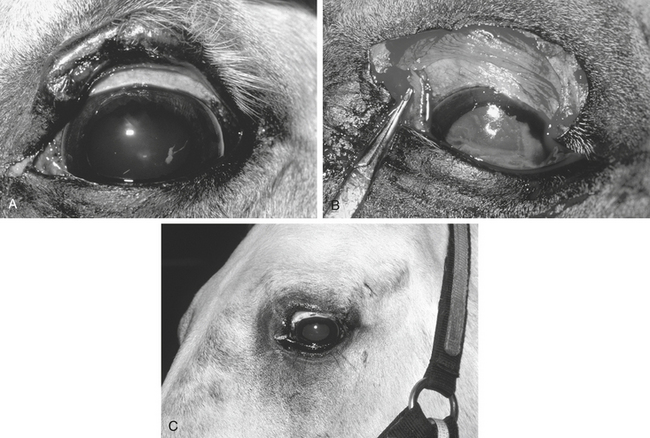
A corneal ulcer is characterized according to size, depth, and the presence or absence of cellular infiltration. In addition, the veterinarian examines the anterior chamber for anterior uveitis. With all corneal ulcers, attempting to establish the cause of the ulceration and eliminate it is esssential (Figure 17-7). The veterinarian examines the palpebral conjunctiva and bulbar surface of the nictitans for the presence of a foreign body, evaluates the blink response and tear film, and obtains a complete history regarding trauma and previous medication. Topical corticosteroids should not be administered in the presence of a corneal ulcer, and a history of previous topical corticosteroid therapy increases the likelihood of infectious, especially fungal, keratitis.
Some specific types of corneal ulcers in the horse include indolent ulcers,36 ulcers with eosinophilic cellular infiltrate,37 collagenase and mycotic ulcers, and possibly viral ulcerative keratitis.3,16
ROUTINE TREATMENT
Treatment of an uncomplicated corneal ulcer involves controlling pain and inflammation, eliminating or preventing infection, and preventing secondary complications (Table 17-3). Healing occurs by migration and mitosis of the adjacent epithelial cells and, depending on the size of the ulcer, should be complete in 2 to 6 days for an uncomplicated corneal ulcer. Complicated corneal ulcers are those that fail to heal in an appropriate time, are secondarily infected, have an ongoing source of irritation or reulceration, have a collagenase component, are associated with corneal vascularization, or are worsening despite appropriate treatment.
Topical broad spectrum antibiotics such as neomycin-bacitracin-polymyxin B ophthalmic are the initial antibiotics of choice. Ointments are preferred for their ease of administration and prolonged corneal contact time. Frequency of administration varies according to severity of the disease. When topical antibiotics are used prophylactically for an uncomplicated corneal ulcer treatment, administration three or four times daily is sufficient, but in severe infectious keratitis, therapy might be hourly. If infection with Pseudomonas spp. is suspected, topical gentamicin, polymyxin B, tobramycin, or preferably ciprofloxacin or ofloxacin is indicated every 2 to 4 hours. Although gram positive organisms predominate initially in equine infectious keratitis, intensive topical antimicrobial therapy results in a significant shift to gram negative organisms and a change in susceptibility patterns.38,39 Additional treatment varies according to the type and severity of the ulcer (see the section on complicated ulcerative diseases). Topical corticosteroids should not be administered to a horse with a corneal ulcer.
Complicated Ulcerative Diseases
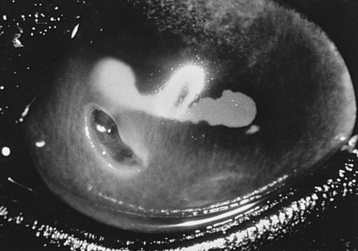
Complicated corneal ulcers are those that require treatment over and above routine ulcer management, are infected, exhibit chronicity or recurrence, are in imminent danger of perforation, or have a collagenase component (Figure 17-8). Secondary infection of a corneal ulcer is suggested by increasing pain, corneal edema, interstitial keratitis associated with an increase in stromal inflammatory cells, corneal vascularization, purulent discharge, severe anterior uveitis, and stromal necrosis and liquefaction.
In many instances, complicated corneal ulcers require frequent and prolonged therapy, and some form of medication delivery system is indicated to ensure adequate treatment. Use of subpalpebral and nasolacrimal medication delivery systems has been described previously.40 The author prefers to use the commercially available Mila lavage catheter (Mila International, Inc., Erlanger, ky.). When a lavage catheter is used, it should be kept clean and medication should be delivered to the eye slowly using air to push the medication through the tubing. Medication used with a subpalpebral lavage system must be in solution, not ointment. The volume of medication used at each treatment time should be 0.1 to 0.2 ml, and air must be used to deliver this medication to the eye. The use of irrigating solution to deliver the medication dilutes the medication. Once daily the system should be irrigated with sterile eyewash to ensure its patency and to rinse away mucus buildup.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree