CHAPTER 23 Equine Infectious Anemia
Equine infectious anemia (EIA), also known as “swamp fever,” is an infectious disease of horses and other equids characterized by recurrent episodes of fever, lethargy, inappetence, thrombocytopenia, and anemia. The clinical signs of EIA were first described in horses in France in 1843,1 and the causative agent was shown to be a filterable agent in 1904.2 This made EIA the first animal disease for which a viral etiology was assigned. During the next 60 years, much descriptive information was obtained in the areas of EIA epidemiology, pathology, and clinical manifestation. However, little progress was made in understanding EIA pathogenesis and immunology, primarily because the virus could not be transmitted to experimental hosts other than the horse, and because the virus could not be cultivated in vitro. Not until 1967 was it demonstrated that field strains of the EIA virus could be propagated in equine leukocyte culture,3,4 and subsequently, several EIA virus strains were adapted to more convenient equine fibroblast culture systems.5,6 These advancements led to significant gains in the knowledge of the EIA virus, its interaction with host cells, and the immunopathogenesis of EIA.
ETIOLOGY
EIAV has a simple ribonucleic acid (RNA) genome that is only 8 kilobases in length. The genome includes three principal genes (gag, pol, env) and three regulatory genes important for viral replication and pathogenesis. The gag gene encodes the structural proteins needed for virus assembly and encapsidation of the genome. These proteins include the nucleocapsid (p11), capsid (p26), and matrix (p15). Gag proteins are the predominant protein components of the EIAV particle.7 The pol gene encodes enzymes required for viral replication (reverse transcriptase) and integration into the host cell genome (integrase). The env gene encodes the virus envelope surface unit (gp90) and transmembrane (gp45) glycoproteins.
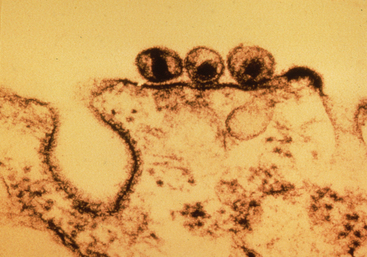
On encountering a host cell, the gp90 glycoprotein (which projects from the surface of the EIAV envelope) binds to a specific receptor on the target cell surface. Recent work indicates that EIAV binds to the newly designated equine lentivirus receptor-1, which is related to the family of tumor necrosis factor (TNF) receptor proteins.8 After binding, the virus envelope fuses with the host cell membrane, and the virion is internalized. Once inside the cell, the EIAV particle uncoats, and replication begins with the production of a double-stranded deoxyribonucleic acid (DNA) copy of the RNA genome, mediated by viral reverse transcriptase. The EIAV DNA is then translocated to the nucleus, and viral integrase inserts the viral DNA into the host cell genome, where it remains as provirus. The integrated provirus utilizes host cell mechanisms for DNA replication, transcription to messenger RNA (mRNA), protein production, and assembly of virus particles. Newly assembled virions bud from the host cell, retaining a portion of the cell membrane as the envelope (Fig. 23-1). The replication cycle is repeated as the new cell-free virus particles encounter and infect new host cells.
Incorporation of the provirus into the host cell genome is a principal mechanism of EIAV persistence within the host. In addition, because reverse transcriptase lacks “proofreading” ability, mutations accumulate in the viral genome during replication. Consequently, a tremendous number of genetic viral variants (“quasispecies”) arise during the course of infection, some of which can escape established immune responses. Antigenic variation and immune escape are therefore major contributing factors to EIAV persistence.
EPIDEMIOLOGY
Prevalence
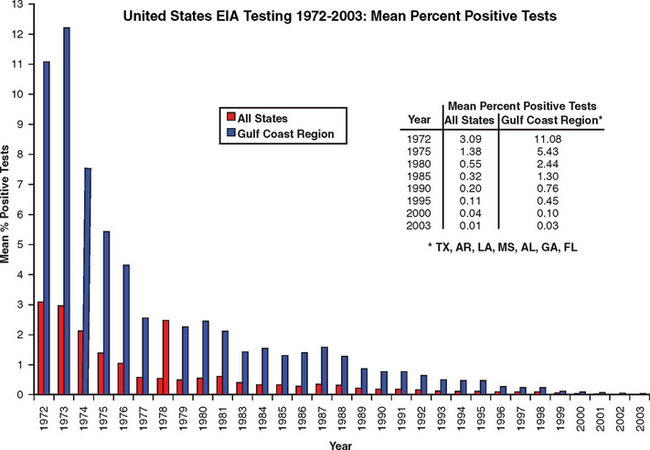
Equine infectious anemia is a worldwide disease of Equidae, including horses, ponies, donkeys, mules, and zebras. Because EIAV is most often transmitted by insect vectors, the prevalence is higher in regions with warm climates. In Brazil, for example, the infection rate in certain horse herds has been as high as 50%.7 In the United States the highest prevalence has occurred in the Gulf Coast region, which has a favorable climate for vector transmission. A reliable serologic test for EIA was developed in the early 1970s,9,10 and this test became the basis for the EIA control program implemented by the U.S. Department of Agriculture (USDA) in 1972. As a result, the prevalence of EIA in the United States has declined from 3.09% in 1972 to 0.01% in 2003, and in the Gulf Coast region the prevalence has declined from 11.08% to 0.03% (Fig. 23-2).
However, these figures do not necessarily reflect the EIA prevalence in the general horse population because they are biased by repeated testing of high-quality horses competing in events that require negative test results. Testing is required only for horses that are entering exhibitions or competitive events, being moved interstate, changing ownership, being imported, or entering auctions or sales markets. According to the USDA National Agricultural Statistics Service, there were 5.25 million horses in the United States at the end of 1997 and 5.32 million at the end of 1998 (the most current data available at this writing).11 According to the USDA Animal and Plant Health Inspection Service (APHIS), 1.37 million and 1.43 million EIA tests were performed in those same years, respectively.12 Based on these data, an average of only 26.5% of the equine population is tested annually. This figure is an overestimate because the tests performed in a given year include repetitive testing of an undisclosed number of horses.
Transmission
Blood from infected horses is the most important source of EIAV for transmission to susceptible horses. Transfer of blood by blood-feeding insects is the predominant means of natural transmission. Because the virus does not replicate within insect cells, insects serve only as mechanical vectors, transferring blood on their mouthparts.13 The most important insect vectors for natural transmission are horseflies and deerflies,7,13–16 both of which are members of the family Tabanidae. Stable flies (Stomoxys calcitrans) have also been shown to transmit the virus,15 but they are less efficient than tabanids as natural vectors. Experimentally, a single horsefly16 and as few as six deerflies14 or 52 stable flies14 can transmit the virus from acutely infected horses to susceptible horses. Studies within the past 25 years indicate that mosquitoes do not transmit EIAV.13,17
Several factors are critical for EIAV transmission by insect vectors. Horses with a high titer viremia and clinical disease are much more likely to transmit EIAV than are inapparent carriers with very low levels of virus in the blood. In one study, horseflies were not able to transmit virus from 10 naturally infected horses with no known history of clinical disease.18 However, the transfer of 1 mL of whole blood from seven of these inapparent carrier horses was sufficient to cause infection in susceptible ponies, and 25 horseflies were able to transmit virus from one inapparent carrier horse that had an acute clinical disease episode 9 months previously.18
Vector population and feeding behavior also influence the probability of transmission. For transmission to occur, the vector must find and feed on an infected horse, be interrupted in that feeding, then find and feed on another horse within a short time. Horseflies are not able to transmit EIAV if the subsequent feeding is delayed 4 hours.16 Tabanids inflict painful bites, contributing to their efficiency as vectors because their feeding is frequently interrupted by defensive movements made by the horse they are biting. Importantly, tabanids have large, slashing mouthparts that sever small vessels, creating a pool of blood. They imbibe blood from this extravascular pool, contaminating their mouthparts in the process (Fig. 23-3). As a result, tabanids transfer a relatively large amount of blood (up to 10 mL) when they feed on a subsequent horse.19

Fig. 23-3 Horsefly (Tabanus fuscicostatus). Note the large mouthparts.
(From Sellon DC: Equine infectious anemia. In Colahan PT, Merritt AM, Moore JN, Mayhew IG, editors: Equine medicine and surgery, ed 5, St Louis, 1999, Mosby, pp 2013-2019.)
The distance separating infected and susceptible horses is another critical factor for transmission. When tabanids are interrupted in their feeding and released 1 foot (30 cm) from the initial host, 87.5% of them will return to the same horse when other horses are tethered 120 feet (36 m) away.20 At a separation distance of 160 feet (48 m), 99% of the horseflies would be expected to return to the original horse if interrupted in their feeding. Therefore, a 200-yard (180-m) separation distance between horses adequately reduces potential EIAV transmission by tabanids. Finally, transmission is more likely when vectors are present in large numbers, explaining the higher EIAV prevalence in regions with warm climates, as well as the increased incidence during the summer months.13
Vertical transmission may occur in utero, at parturition, or after ingestion of infected colostrum or milk.21–23 Transplacental transmission appears to be a rare event and is more likely when the mare develops acute clinical disease and high-titer viremia during gestation. In one study, 12 of 52 foals from EIAV-infected mares were virus positive (as determined by blood inoculation into susceptible ponies) when tested at 1 week to 6 months of age.21 Although 7 of the 12 foals were offspring of mares that had clinical signs of EIA during gestation, transplacental transmission was confirmed in only one aborted fetus.21 In other studies, 18 of 20 foals born to infected dams were virus negative (as determined by pony inoculation) at weaning,24 and 29 of 31 foals from infected mares had no evidence of infection at weaning.25 In virus-negative foals born to infected dams, EIAV-specific colostral antibody can be detected in serum for 25 to 195 days.24,25 More recently, of 12 foals born to EIAV-infected mares in a herd of wild horses in Northeastern Utah, none was found to be virus positive by reverse transcriptase–polymerase chain reaction (RT-PCR). However, EIAV-specific colostral antibodies could be detected in the serum from these foals for up to 336 days.26
Venereal transmission is possible because semen from infected stallions can result in infection after subcutaneous inoculation.22 However, breeding EIAV-infected stallions to uninfected mares did not result in transmission except for one possible case in which a mare sustained a vaginal laceration.22 Finally, EIAV can be transmitted iatrogenically through transfusion with contaminated blood products and by the use of blood-contaminated materials, such as surgical and tattooing instruments, hypodermic needles, and dental equipment. The virus is known to survive for up to 4 days on hypodermic needles held at room temperature.17
PATHOGENESIS
Infection with EIAV can result in a variety of clinical, clinicopathologic, and pathologic abnormalities, including fever, lethargy, inappetence, thrombocytopenia, anemia, splenomegaly, hepatomegaly, lymphadenopathy, weight loss, dependent edema, and hemorrhage.7,27–29 Clinical disease severity correlates with viral load, and thus depends on the level of virus replication. The virus infects macrophages, and most infected macrophages are detected in the spleen.30 Although peripheral blood monocytes are infected, viral replication occurs primarily in mature tissue macrophages, which serve as the predominant source of the high-titer viremia during acute infection.31 Tissue macrophages are also the primary cellular reservoir for EIAV during subclinical infection.32 Although viral replication is restricted during periods of clinical quiescence, the virus continues to replicate at all times.32 The spleen contains the highest level of replicating virus during acute infection, but other tissue sites of active infection include the liver, lymph nodes, bone marrow, peripheral blood mononuclear cells, lung, adrenal gland, kidney, and brain.31–33 During inapparent infection, replicating virus can be detected in most of these same tissues, but at much lower levels.32,33 Endothelial cell infection also occurs in EIAV-infected horses.34
Although the immune response to EIAV is critical for the eventual control of viral load and clinical disease, immunologic mechanisms are involved in the development of lesions and clinical disease. The infection and destruction of macrophages, as well as the observation that most of the infectious virus in the serum of infected horses occurs in the form of immune complexes,27,28,35 are important factors in the immunopathogenesis of EIA. Infection of macrophages with virulent EIAV induces the upregulation of TNF-α, interleukin-1 (IL-1), and IL-6.36 Elevation of the circulating levels of these proinflammatory cytokines most likely contributes to the fever, lethargy, and inappetence observed during acute disease.36–39
Thrombocytopenia
Thrombocytopenia is one of the earliest and most consistent detectable abnormalities in EIAV-infected horses and closely correlates with fever and viremia.29 The pathogenesis of EIAV-induced thrombocytopenia is multifactorial. An increase in platelet-bound immunoglobulin G (IgG) and IgM during episodes of thrombocytopenia in experimentally infected horses suggests an immune-mediated mechanism.40 However, foals with severe combined immunodeficiency (SCID) develop the same degree of thrombocytopenia as immunocompetent foals after EIAV infection.41 Foals with SCID lack functional T and B lymphocytes and cannot mount antigen-specific cell-mediated or antibody responses.42,43 In addition, platelet production during EIAV infection is significantly reduced in both SCID and immunocompetent foals.41 These results indicate that suppression of platelet production is likely the predominant mechanism of EIAV-induced thrombocytopenia. Serum activities of potential negative regulators of thrombopoiesis, including TNF-α, TGF-β, and interferon-γ (IFN-γ), are increased just before, and at the onset of, thrombocytopenia in acutely infected horses.39 Both TNF-α and TGF-β in plasma from EIAV-infected horses suppress equine megakaryocytes in vitro.44 Additional work indicates that platelets become activated and hypofunctional in acutely infected horses, and as a result, platelet aggregates may form, which are then removed from the circulation.45 This could represent a nonimmune mechanism of platelet destruction in EIAV-infected horses. Finally, infection of endothelial cells could promote platelet adherence and aggregation, leading to thrombocytopenia.34
Anemia
Anemia is a consistent clinical abnormality associated with EIAV infection, and its severity directly correlates with the frequency and duration of febrile episodes.28 Similar to thrombocytopenia, the pathogenesis of anemia is multifactorial and includes immune-mediated erythrocyte destruction as well as decreased erythropoiesis. Early work indicated that erythrocyte life span is reduced to between 28 and 87 days (normal mean, 136 days) in EIAV-infected horses, and that both intravascular hemolysis and extravascular hemolysis occur.46
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree