Host species
Subtypes of influenza A viruses
Human
H1N1, H2N2, H3N2, H5N1, H9N2, H7N7, H7N2, H7N3, H10N7, H7N9, H10N8
Horses
H3N8 and H7N7
Pigs
H1N1, H1N2, H3N2, H1N7, H3N1, H4N6, H2N3 and H9N2
Fowl
1–16 HA types and 1–9 NA types
Dogs
H3N8, H3N2, H5N1
Cats and tigers
H5N1
Seal
H7N7 and H4N5
Mink
H10N4
Bats
H17N10 and H18N11
The mode of transmission of influenza viruses includes direct transmission between an infected host and susceptible host, the airborne route through the aerosols produced by infected host or indirectly through contaminated surfaces (Hall 2007; Weber and Stilianakis 2008). The clear-cut demarcation among the route of transmission is not very clear, and all the three mode of transmission may result in the spreading of the virus (Tellier 2006). A single sneeze results into release of around 40,000 droplets (Cole and Cook 1998) and inhalation of just a single droplet may cause the disease (Weber and Stilianakis 2008). The survival of influenza viruses in the droplets depends upon the relative humidity and sunlight. Therefore, low humidity and poor sunlight in the winter helps the virus survival in airborne droplets and helps in its transmission (Weber and Stilianakis 2008). The interspecies transmission of influenza viruses is shown in Fig. 5.1.
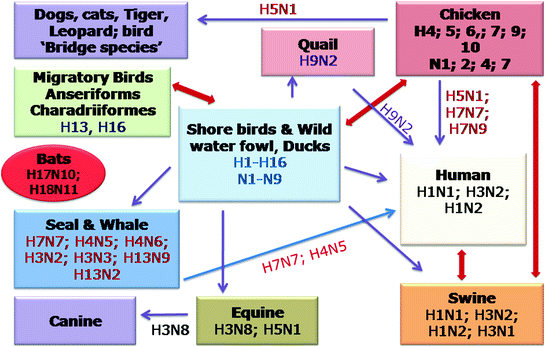

Fig. 5.1
Interspecies transmission of influenza A viruses
5.1 Avian Influenza (Bird Flu)
AI, also known as avian flu/bird flu/fowl plague/fowl pest/chicken ebola, was relatively uncommon until 1997, but during the past 15 years its incidence has increased steadily. Between 1959 and 1998 AI outbreak affected 23 million birds, whereas it reached >200 million from 1999 through 2005. It is the most fearful viral disease of birds, particularly affecting domesticated birds with very high flock mortality (up to 100 %). Since the late 1990s, AI outbreaks pose great public health problems, especially through devastating consequences for the poultry industry, creating negative awareness among the public and the risk of generating a pandemic virus against mankind through an avian–human link.
AI, caused by the genus Influenzavirus A, consists of 18 HA and 11 NA subtypes, present mainly in wild waterfowl, shorebirds and gulls, and some constrained subset found in birds, human beings and other mammals (Wainwrighta et al. 2012; Brown et al. 2013; Wibawa et al. 2014; Wille et al. 2014). The H17N10 and H18N11 has been isolated only from bats (Tong et al. 2012, 2013). The H5 and H7 are responsible for generalized disease while the remaining subtypes are associated with localised form of the disease. Although AI is primarily a disease of domesticated poultry such as chickens, turkeys and pheasants, subclinical infections do occur in a wide range of feral migratory birds. Fowl act as the natural asymptomatic carriers of AI virus whereas major reservoirs for the virus are ducks, geese as well as migratory birds that play an important role in the disease transmission to domestic birds mainly through respiratory secretions and droppings (Swayne and Halvorson 2003; Arzey 2004; Campitelli et al. 2004; Musa et al. 2009; Stallknecht and Brown 2013). The high moisture and low temperature conditions favour its survival in poultry manure for more than 100 days. It has been reported that one million susceptible birds may become infected with 1 g faeces of the infected bird (Swayne and Halvorson 2003).
The disease is highly contagious and can spread to vast geographical areas very fast if strict biosecurity, prevention and control measures, etc., are not properly followed. From the infected birds, the viruses get excreted through their respiratory tract, conjunctiva and faeces and spread through direct contact as well as indirectly by means of aerosols, virus contaminated soil, feed, water, faeces, equipments, fomites, etc. (Alexander 2001; FAO 2004; de Jong et al. 2005; WHO 2006; Vong et al. 2008, 2009; Kandun et al. 2009). Another possible means of transmission is from the blood or body fluids of infected birds via slaughterhouse and other food processing practices (Greiner et al. 2007), consumption of uncooked poultry products, improper waste disposal practices, etc. (Beigel et al. 2005; de Jong et al. 2005; Apisarnthanarak et al. 2005; Gambotto et al. 2008; Coburn et al. 2009).
Subclinical infection has been recorded in South-East Asia in geese and ducks due to involvement of H5N1 virus. In Hong Kong, in May, 2001, increase in mortality has been recorded severely in chicken due to which a decision was made immediately to cull over a million birds within a single month, which resulted in no further report of poultry cases (Guan et al. 2002). Usually, in the winter months outbreaks have been recorded in poultry in Hong Kong due to poultry meat import in order to meet the requirements of lunar New Year activities (Smith et al. 2006). Although HPAI outbreak in wild birds is rare, there is discovery of highly pathogenic H5N1 cases in wild birds through surveillance. In free flying wild waterfowl, high mortality rates have been observed having consistent genetic marker with prior adaptation in land-based poultry birds (Ellis et al. 2004; Lui et al. 2005). Results based on laboratory diagnosis have revealed many genotypes of H5N1 that are in circulation in Hong Kong among wild birds. In southern China during 1999–2002, surveillance of AI in water fowl yielded several H5N1 isolates from ducks that were apparently healthy (Chen et al. 2004; Cowling et al. 2013). Shedding of virus has been recorded from trachea and cloaca in ducks causing death in inoculated chickens (Hulse-Post et al. 2005).
The movement of infected birds is responsible for the outbreak of low pathogenic avian influenza (LPAI) in commercial poultry. Along with this, some other factors like dirty or improperly cleaned crates and contaminated vehicles from the live bird market system (LBMS) to the poultry farms also play important roles in the spread of the virus. In North America, surveillance studies have shown that there is repeated recovery of influenza A viruses in waterfowl and shorebirds but the recovery of virus is influenced by geography and season, age and species of birds. In certain parts of the United States the LBMS has been considered as a reservoir made by man for influenza viruses in addition to the natural reservoirs of the virus (Senne et al. 2006; Stallknecht and Brown 2007). The role of live bird markets (LBM) in the epidemiology of AI was revealed in the 1997 Hong Kong epidemic (Sims et al. 2003; Kung et al. 2007). Since then several researchers have reported that H5N1 viruses circulated among geese, ducks and chickens in LBMs in affected Asian countries since 2003 (Nguyen et al. 2005; Webster et al. 2006; Jadhao et al. 2009; Liao et al. 2009; Van Kerkhove et al. 2009; Indriani et al. 2010; Wan et al. 2011; Sarkar et al. 2012). Additionally, many festivals associated with raising (either commercially or domestically) (Vong et al. 2009), selling and transporting the poultry birds also plays a role in spreading of the virus in Africa (Musa et al. 2009) and Thailand (Ortiz et al. 2007; Hinjoy et al. 2008; Tiensin et al. 2009; Santhia et al. 2009). Interspecies transmission among chickens, turkeys and wild birds, particularly ducks, may play an important role to introduce avian influenza viruses in susceptible flocks (Nagarajan et al. 2012). Influenza in turkeys is more common in countries where birds are kept in an environment to which wild birds have easy access.
Due to restriction on control and export due to notifiable viral infections, the ostrich industry in South Africa was found to be badly affected. A syndrome of green urine was first observed by the farmers in the early and mid-1980s. Local migratory water birds play an important role in spread of AI as revealed by surveys due to the involvement of free-range type of production systems. Wet and colder months are more favourable for survival as well as detection of virus. The severity of the disease is governed by management and population density, immune status as well as age (Hanson 2002; Olivier 2006).
Aquatic birds are considered the prime source of influenza viruses and this perpetuation in aquatic avian species along with its ecological features has been studied by the phylogenetic analysis of RNA segments coding for the spike proteins (HA, NA and M2) and the internal proteins (PB2, PB1, PA, NP, M and NS) of influenza A virus isolated from different host range and geographical regions. The study revealed the following facts regarding the influenza A virus subtypes:
Migrating waterfowl and shorebirds harbours influenza viruses with all the known HA and NA subtypes and these act as two partly overlapping reservoirs of this virus.
There are a number of geographical lineages to the Influenza viruses, exemplified by the NP gene. Examples for the host-specific lineages include equine Prague/56, other recent equine strains, all avian strains, H13 gull strains, classical swine and human strains.
All mammalian influenza A and B viruses originated from the avian gene pool and the perpetuation of the virus in aquatic birds is due to the low-level transmission within that species throughout the year.
Amidst the host-specific virus lineages, periodic exchanges of this virus genes or whole viruses occur between species, resulting in pandemic diseases to mammals and birds. There is evidence that most recent human pandemic for influenza originated in southern China.
Some of the studies point out swine as the intermediate host for the genetic exchange of influenza viruses among different species but not with ample evidence.
Studies on the ecological properties of influenza viruses have now enabled the interdiction of new influenza viruses into humans (Webster et al. 2006).
5.1.1 Important Historical Events
1878—‘Fowl plague’ reported for the first time.
1880—‘Fowl plague’ differentiated from fowl cholera on the basis of clinical and pathological features.
1901—The cause of fowl plague determined to be a filterable agent.
1934—Propagation of causative agent in embryonated chicken eggs.
1955—Causative agent classified as influenza A virus.
1959—Isolation and identification of HPAI virus of H5 subtype (A/chicken/Scotland/59) (H5N1).
1961—Isolation of AI virus from wild birds [A/tern/South Africa/61 (H5N3)].
1970—Discovery of huge reservoirs of influenza viruses in water fowls.
5.1.2 HPAI Disease Outbreaks
1894—A severe disease outbreak of HPAI occurred in Italy and was disseminated via chickens to Austria, Germany, Belgium and France.
Early twentieth century—HPAI was reported in Switzerland, Romania, Russia, Netherlands, Hungary, Great Britain, Egypt, China, Japan, Brazil and Argentina.
1924–25 and 1929—HPAI was reported in the United States.
In midtwentieth century—HPAI was reported from Europe, North Africa, Middle East, Asia, Russia and American continents (Swayne and Halvorson 2003).
1957—A serious pandemic had been reported to be caused by H2 subtype of Influenza A virus having two lineages: American and Eurasian (Makarova et al. 1999).
1959—H5N1 in chickens in Scotland (Pereira et al. 1965).
1961—H5N3 in South Africa, 1,300 common terns affected (Becker 1966).
1963—H7N3 in England, 29,000 turkeys affected (Wells 1963).
1966—H5N9 in Ontario, 8,100 turkeys affected (Lang et al. 1968).
1976—H7N7 in Victoria, 42,000 chickens and 16,000 ducks affected (Turner 1976).
1979—H7N7 in chickens in Germany and in turkeys in England (Alexander and Spackman 1981; Alexander 2000a).
1983—H5N2 caused an epidemic among 17 million birds in Pennsylvania and New Jersey, United States (Swayne and Suarez 2000). H5N8 was also reported in turkeys in Ireland (McNulty et al. 1985).
1985—H7N7 outbreak occurred in Victoria, Australia and affected more than 2 lakh chickens (Barr et al. 1986).
1991—H5N1 in England, 8,000 turkeys affected (Alexander et al. 1993).
1992—H7N3 in Victoria, Australia, 4,300 chickens and 5,700 ducks affected (Selleck et al. 1997).
1994—H5N2 caused mass destruction of chickens in Mexico (Swayne and Halvorson 2003).
1995—H7N3 affected more than 20,000 layer chickens in Queensland, Australia (Swayne and Halvorson 2003) and about 3 million birds in Pakistan (Muhammad et al. 1997).
1997—H5N1 outbreak among 3.2 million chickens in Hong Kong and it was the first report on human transmission of HPAI (Suarez et al. 1998; Shortridge 1999; Tam 2002). In the same year H7N4 virus outbreak occurred in New South Wales, Australia, affecting 1,61,000 chickens and 261 emu (Selleck et al. 2003). Also, a total of eight outbreaks of H5N2 occurred in Italy affecting a poultry population of more than 5,000 (Swayne and Halvorson 2003).
1999—H7N1 caused devastating epidemic in Italy, 10.5 million chickens, 2.7 million turkeys and nearly 5 lakh guinea fowls, quails, ducks, pheasants and ostriches were involved in the outbreaks (Pascucci 2000; Capua et al. 2003).
2001—H5N1 outbreak occurred in Hong Kong that affected about one million birds (Tam 2002; Swayne and Halvorson 2003). H7N3 reported in Pakistan also.
2002—H5N1 reported in Hong Kong that led to the mass depopulation of the flocks in the region (Ramirez et al. 2004).
2003—H5N1 outbreaks occurred in Hong Kong in chickens (Ramirez et al. 2004). An epidemic of H7N7 also occurred in Netherlands affecting 255 flocks that led to the culling of 30 million birds (Stegeman et al. 2004; Wit et al. 2004). Korea also experienced H5N1 outbreak in poultry.
2004—H5N1 infection in poultry was reported for the first time in Thailand (Simmerman et al. 2004). Later, Indonesia, Cambodia, Vietnam, Korea, Japan, Malaysia and China also reported the presence of H5N1 virus. H7N3 outbreaks reported in poultry flocks of Canada (Hirst et al. 2004; Tweed et al. 2004), Chile (Suarez et al. 2004) and Pakistan. Laos experienced H5 subtype outbreaks (OIE 2005).
2005—H5N1 outbreaks reported in most of the South-East Asian countries, Russia, Romania, Turkey, Croatia and Ukraine. In addition, H5N1 has been detected in birds smuggled into Taiwan (Taipei China) (OIE 2005).
These data point out that HPAI viruses cause fatal clinical signs and mortality in closely related Galliformes, viz. chickens and turkeys, while other closely related group of birds (ducks, geese, etc.) had lesser consequences of the viruses.
Thus, AI outbreaks have been reported worldwide from time to time. H7 subtypes (H7N1 and H7N7) were the main culprits behind the HPAI outbreaks that happened in the first half of the twentieth century. In the later part, H5 subtype made its world debut in 1959 in Scotland and since then has caused the maximum number of casualties in birds (Swayne and Suarez 2000; Alexander 2000b, 2001, 2003; Hafez 2003; Swayne and Halvorson 2003; OIE 2005). The number of outbreaks of AI seems to be increasing over the last 10 years and many more different AIV subtypes are being reported in poultry worldwide. The epidemiological trend of AI with its constant emergence, highly pathogenic and pandemic nature, especially in Asian and European countries, has caused havoc among the poultry industry all over the world.
AI infection caused by two subtypes, viz. H5 and H7 had been recorded in poultry ever since the 1990s across a large area of the world. LPAI H9N2 has spread across the entire continent of Asia in that particular time. This has given rise to influenza endemicity in several countries that were affected previously. However, there is a tendency of overshadowing of these outbreaks due to the emergence of H5N1 HPAI virus globally. After initial isolation in China it has now spread throughout Asia and into Europe as well as Africa in both poultry and wild birds. This has resulted in the death and culling of millions of poultry that has possessed a zoonotic threat significantly (Alexander 2007). With regard to health and welfare of animals, supply of food, economies as well as biodiversity there is major implication of H5N1 viruses. On surveillance of both LPAI as well as HPAI viruses in both animals and humans, the main focus should be on interventional strategies. This provides warning systems early along with repositories of virus allowing the establishment of seed viruses as vaccine candidates in time (Osterhaus et al. 2008).
Recently, AIV has caused chaos in the poultry industry leading to enormous economic losses worldwide (Elci 2006; Musa et al. 2009; Nayak et al. 2010; Wainwrighta et al. 2012). Enormous economic losses are encountered globally due to AI creating chaos worldwide (Alexander 2007; Dhama et al. 2005; Kataria et al. 2005). More than 60 countries are affected by the H5N1 strain of AI particularly, incurring losses of more than 400 million birds and 379 human lives. This indicates that in the recent years the flu virus is becoming more and more dangerous (Alexander 2008; OIE 2008; Adams and Sandrock 2010). From the end of 2003 till 2013, AI has been reported in the following countries: Vietnam and Egypt; Thailand, Indonesia and Myanmar; Bangladesh; Romania; Russia; Korea and China; India and Pakistan; Nigeria. Till March 12, 2013 the south Asian regional countries, viz. Bangladesh and Bhutan; Cambodia and Hong Kong; India and Nepal have reported the outbreak of AI (Ellis et al. 2004; Kwon et al. 2005; WHO 2011; Tiwari and Dhama 2012; OIE 2013). The most number of AI cases worldwide have been reported from the following countries: Vietnam (2681), Thailand (1141), Egypt (1084), Bangladesh (548), Romania (273), Indonesia (261), Turkey (219), Russia (149), Myanmar (115), Korea (Rep. of) (112), China (People’s Rep. of) (106), Nepal (101), India (97), Nigeria (65), Pakistan (51), Ukraine (42), Cambodia (37), Japan (32), Saudi Arabia (29), Afghanistan (22), Kuwait (20), Laos (19), Bhutan (19), Sudan (18), Hong Kong (P.R. China) (17), Malaysia (16), Israel (15) and Poland (10) (WHO/OIE/FAO H5N1 Evolution Working Group 2012).
5.2 Swine Influenza
Swine influenza is one among the primary porcine respiratory diseases (Brown 2000; Heinen 2003; Dhama et al. 2012) inflicting high morbidity but with low case fatality rate (Vincent et al. 2009). Since pig is susceptible to both human and avian influenza viruses, it can be an important host in disease ecology. The primary strains of influenza A viruses causing the disease include H1N1, H1N2 and H3N2 (Brown et al. 2012). Swine influenza was first reported in 1918 from US, Hungary and China along with a human pandemic influenza causing a worldwide mortality of 20 million (Chun 1919; Koen 1919; Beveridge 1977; Brown 2000) and later the presence of a common ancestor for porcine and human influenza virus A has been proven (Gorman et al. 1991; Kanegae et al. 1994; Reid et al. 1999). The most prevalent subtypes of Influenza A viruses infecting pigs are H1N1 (including classical swine H1N1 and avian-like H1N1) and H3N2 (human- and avian-like H3N2). It is endemic in swine populations worldwide and the increasing incidences are based on factors like antigenic shift, cold weather (more outbreaks in the colder months due to low humidity and poor sunlight) (Easterday 1980), lack of proper husbandry practices and other secondary infections. The disease can remain enzootic in farms particularly by young pigs and get transmitted mainly by aerosols, fomites as well as carriers like waterfowl and humans (Easterday and Van Reeth 1999). Over 80,000 influenza gene sequences have been published by the Influenza Genome Sequencing Project and other contributors from the isolates of numerous species around the world, leading to advances in molecular influenza epidemiology (Nelson and Holmes 2007). These studies have demonstrated a high frequency of genetic reassortment in A/H3N2 viruses, especially among the genetically distinct cocirculating dominant and subdominant lineages of previous seasons, resulting in new antigenic variants (Holmes et al. 2005; Nelson et al. 2006; Vasoo et al. 2009; Lee et al. 2011). In North America, Europe and Asia two genes have been recognized in the H1N1 subtype of swine influenza along with influenza virus genes of avian and human origin. This is regarded as a quadruple reassortant virus acquiring the capacity of man-to-man transmission. In recent years H1N1 swine flu virus (H1N1 triple human/avian/swine reassortant virus) have caused human pandemic (Dhama et al. 2012). The H3N2 virus currently has shown a novel reassortment from swine with confirmed cases in human affecting children mostly in United States (Skowronski et al. 2012). In the context of the concept of ‘original antigenic sin’ it has been postulated that if there is an exposure of a child to an influenza virus for the first time there is development of strongest immunity in the later generations to come. As a result of this, there may be development of natural immunity to the A/H1N1 pdm pandemic virus that is circulating at the moment (Chowell et al. 2011; Rifkin and Schaal 2012).
5.3 Equine Influenza
The most destructive outbreak of equine influenza is ‘The Great Epizootic of 1872’ occurred in North America, leading to a major economic panic in US for 6 years. Thereafter, epidemics of equine influenza occurred in Europe and North America during 1956 (H7N7), 1963 (H3N8), 1969, 1979, 1989 and 2007 (significantly affected the horse racing industry in Australia). The virus mainly spreads through inhalation of infected droplets and nasal discharge (Radostits et al. 2006) as well as through contaminated transport vehicles and other equipments. Infected horses excrete the virus in their exhaled air before they show any signs up to 8 days after initial infection, but recovered horses do not become carriers (Timoney 1996; Morley et al. 2000). There are evidences for natural case of mixed infection, suggesting that the virus infects other species and the donor equine host may possess the genetic diversity required for the virus adaptation to new host species. The unpredictable nature of the equine influenza virus H3N8 has been reported (Daly et al. 2011). Equine influenza virus can infect the human beings but the symptoms are very mild and mostly remain subclinical but may represent a potential biohazard to laboratory personnel (Alexander and Brown 2000). After a gap of nearly 20 years, equine influenza virus outbreak by H3N8 subtype was reported from India in 2008 from different regions. Molecular analysis revealed that its haemagglutinin gene was closely related to Clade 2 of the Florida sublineage in American lineage (Virmani et al. 2010).
5.4 Canine and Feline Influenza
Pets like dogs and cats have been domesticated by humans since at least 3,500 years and there is always a positive trend for keeping them as pets (Kumar et al. 2012a,b; Singh et al. 2013). Along with the advantages and benefits particularly as pets, dogs and cats may also serve as source of zoonotic diseases and the epidemiology of influenza infections in dogs and cats have been attaining considerable attention during the last decade (Verma et al. 2008). Canine and feline influenza is an infectious disease of the upper respiratory tract of dogs and cats, respectively, caused by influenza A virus (H3N8) (Rahman 2012). Canine influenza virus (CIV) is a recently identified, extremely contagious respiratory pathogen of dogs, inflicting high morbidity, low mortality and a case fatality rate of 5–8 % in greyhound dogs and less than 1 % in the general pet population (de Morais and Helio 2006).
The first report of the disease under natural condition was in greyhound dogs of Florida in 2004, after conducting dog racing on horse racing track (Dubovi and Njaa 2008). Now it is proved that canine influenza originated from equine influenza virus and jumped species in 1990. Since then, H3N8 was also responsible for a major dog flu outbreak in all breeds of dogs (Tremayne 2006) including pet dogs. At present the US is considered an endemic area for the presence of canine influenza virus (Yin 2007). Additional subtypes of influenza virus reported to be infecting dogs include H3N2 (avian-origin) from South Korea (Song et al. 2009; Lin et al. 2012) and southern China in 2007 (Li et al. 2010) and Brazil in 2012 (Mancini et al. 2012), HPAI H5N1 from south to east Asia in 2006 and the new North American H1N1 from New York in 2009. So far there is no report of its transmission to humans and other animals (Crawford et al. 2006). Heterogeneity of these viruses in their natural reservoirs and recent evidence on the establishment of natural clinical infections in carnivores necessitates that this infection should also be considered in canines and felines with lower respiratory diseases. Also, the possible epidemiological role of canine and feline species in interspecies transmission needs to be explored (Harder and Vahlenkamp 2010). Equine Influenza virus H3N8 was found to be transmitted from horses to dogs coming in close proximity to infected horses in Australia in 2007 (Kirkland et al. 2010; Crispe et al. 2011). However, dogs experimentally infected with canine influenza A H3N8 virus were unable to transmit infection to horses cohoused with these infected dogs (Yamanaka et al. 2012). The annual prevalence of influenza in dogs due to H3N8 in USA increased from 44 % in 2005 to 62 % in the year 2007, before decreasing to 38 and 15 %, in 2008 and 2009, respectively (Anderson et al. 2013). A seroprevalence for canine influenza virus H3N8 of 1.9 % in flyball dogs (Wiley et al. 2013), and 0 % in racing sled dog (Pecoraro et al. 2012) was reported.
5.5 Human Influenza (Flu)
In humans, influenza (flu) occurs either as an epidemic or as a pandemic (Nicholson 1998) and the infection is mainly from influenza viruses subtypes A, B or C that can be differentiated by serological tests using conserved viral nucleoprotein or matrix protein (Beard 1970). Recent human influenza outbreaks were inflicted by the antigenic variants of influenza A viruses (H1N1, H3N2 or their reassortant H1N2) and influenza B viruses. Genomic analysis of H1N1 subtype that occurred in Mexico in 2009 revealed its close relation with the common swine influenza A reassortant viruses found in Asia, North America and Europe (Trifonov et al. 2009). The clinical illness of human influenza include that for usual flu, viz. cough, sore throat, fever and in fatal cases leading to breathing problems as well as pneumonia. Immune status of the victim plays a major role in determining the severity of the infection and previous exposure of the virus renders partial immunity to the host. That is why there are severe clinical signs as well as higher death rate in young ones and emergence of new influenza A virus subtypes results in pandemic form of influenza. In those infants whose maternal antibodies have declined, exposure to an influenza agent may lead to an attack rate of 30–50 % (Noble 1982; Glezen et al. 1977).
Transmission of human influenza is mainly through aerosols (Tellier 2006) affecting all ages especially pregnant women (Steinhoff and MacDonald 2012) and those below 30 years (Wilde 2010). The incubation period for influenza A and B viruses is 3 and 4 days, respectively. The incidence of influenza is more during winter in temperate countries (Wilde 2010), whereas it is more common during winters and rainy seasons in tropical and subtropical countries.
Bird Flu in Humans Bird flu is an infectious disease affecting birds and was first observed in Italy in 1878. Although the bird flu influenza virus A usually does not affect humans but there are reports proving the infection of bird flu virus in human (Morens et al. 2009; Zimmer and Bruke 2009). Severe life-threatening complications are characteristics of bird flu in humans (Malik Peiris 2009; Riquelme et al. 2009). Juveniles and migrants play an important role in the seasonal epizootics of avian influenza virus (Dijk et al. 2014).
In 1997, HPAI was first reported in Hong Kong where 18 people were hospitalized and 6 died. Investigation confirmed the spread of virus from birds to humans, and about 1.5 million chickens were killed to remove the source of the virus.
In 1999, LPAI A (H9N2) virus was isolated from two children in China and Hong Kong. The source of infection was infected birds.
In 2002, one person in Shenandoah Valley, Virginia, was found serologically positive for H7N2 virus infection after its outburst among poultry.
In 2003, two cases of HPAI H5N1 were recorded in a Hong Kong family which had travelled to China and one person among them died.
In 2003, H7N7 infection was reported among 89 people in Netherlands, which was linked with an earlier outbreak of H7N7 influenza virus among poultry.
In 2003, LPAI H9N2 was confirmed in a child in Hong Kong, who later recovered.
In 2003, an influenza virus H7N2 was confirmed from a person showing clinical signs of respiratory illness in New York.
In 2004, highly pathogenic avian influenza A (H7N3) was confirmed from poultry workers after an outbreak of this virus among poultry in Canada.
In January 2004, WHO first time reported the incidence of H5N1, a highly pathogenic influenza A virus, in Asia with the disease spreading to humans in Vietnam and Thailand.
In 2005, the human form of the disease spread to Cambodia and Indonesia and around the world.
By 2012 it had infected millions of poultry farmers and human beings, caused approximately $20 billion economic loss, and spread to almost 50 countries including Britain, Nigeria, Afghanistan, Israel, Cameroon, Iraq, Myanmar, Thailand, China, Egypt, Indonesia, Cambodia, Vietnam, Bangladesh and India.
Pandemic influenza is associated with a shift in the mortality age of host and this can be explained by recycling and immune pathology (Andreasen et al. 2007). According to the recycling hypothesis, if the pandemic virus possesses a haemagglutinin antigen which has not circulated in human populations previously, then the older people will be at a greater risk (Palese 2004). But for the same case, the immune pathology hypothesis explains that the older individuals will be on immune senescence so that younger ones will be at higher risk (Bermejo-Martin et al. 2007).
Morbidity and mortality due to avian-origin H7N9 strains was reported for the first time in China and Taiwan in 2013. The majority of the cases of H7N9 infection were observed in older adults. Difference was observed between urban and rural areas. The risk of getting infected with H7N9 in men compared to women was more in urban areas, whereas rural men and women were equally susceptible (Cowling et al. 2013; Yu et al. 2013; Zhuang et al. 2013). The source of H7N9 virus that infected humans was H7N9 virus outbreaks among chickens in live poultry markets (Bao et al. 2013; Chen et al. 2013; Shi et al. 2013; Tang and Chen 2013).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


