CHAPTER 44 Enteric Clostridial Infections
ETIOLOGY
The genus Clostridium comprises a diverse group of gram-positive, anaerobic, spore-forming bacteria (see Chapter 27), many of which have been implicated as pathogens of a variety of body systems in different animal species. In particular, Clostridium difficile and Clostridium perfringens are important enteropathogens and in some areas are the most frequently identified causes of colitis in horses.1–7
Clostridium difficile is the most commonly diagnosed cause of antimicrobial-associated and nosocomial diarrhea in humans.8 (Figs. 44-1 and 44-2). It has received increasing attention over the past few years because apparent changes in the incidence and severity and large outbreaks of nosocomial disease have been reported.9,10 In horses, a variety of studies have implicated C. difficile as a cause of enterocolitis in adult horses and foals,1,2,4–7,11–13 and disease has been reproduced experimentally in immunocompetent foals.14 C. difficile can be found throughout the environment in veterinary hospitals and horse farms.15–17 Outbreaks of C. difficile–associated disease (CDAD) have been reported in an equine hospital and on farms.5,6,18
Although of lesser importance than C. difficile, C. perfringens is a recognized cause of nosocomial and antimicrobial-associated diarrhea in humans19 (Fig. 44-3). It is a leading cause of food poisoning through production of an enterotoxin in improperly stored foods.20 C. perfringens is commonly found in the intestinal tracts of a variety of animal species and in the environment, and it has been described as being the most widely occurring pathogenic bacterium.21 It is also a recognized cause of enteric disease in horses of all ages.3,4,7,22–25
A variety of other clostridia, including Clostridium sordellii and Clostridium septicum may also be involved in equine colitis;26,27 however, minimal information is currently available. Clostridium spiroforme and Clostridium colinum are causes of enteric disease in other species, but their role in equine disease, if any, is unknown.21 Implication of other clostridia in equine diarrhea is hampered by limitations in specific diagnostic tests and difficulties in determining whether a clostridial species is part of the normal microflora, has overgrown in response to flora disruption from diarrhea of another etiology, or is the primary etiologic agent.
EPIDEMIOLOGY AND PATHOGENESIS
Clostridium difficile
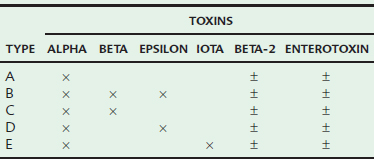
The pathogenesis of CDAD in horses presumably occurs from proliferation of toxigenic strains of C. difficile in the intestinal tract, with subsequent toxin production and development of enterocolitis. C. difficile can produce a variety of toxins. Toxin A, an enterotoxin, and toxin B, a cytotoxin, have been evaluated most extensively and are believed to work synergistically to cause disease (Table 44-1). The vast majority of toxigenic C. difficile isolates produce both these toxins; however, a small percentage of isolates from humans are toxin A negative and toxin B positive but are able to produce disease.28 A toxin A–negative, B–positive strain was recently isolated from a horse with duodenitis and proximal jejunitis (L. Arroyo, personal communication). Some isolates, including those from horses, also produce a binary toxin (CDT), but the role of this toxin in disease is currently unclear.29,30 Not all strains of C. difficile are able to cause disease; approximately 13% of equine isolates cannot produce any known toxins and are clinically irrelevant.31
A small percentage of normal horses may carry toxigenic strains of C. difficile without adverse effects, although the prevalence of subclinical carriage in normal adult horses is quite low (0%-1%).2,7,15,16 Antimicrobial therapy may influence colonization rate, as demonstrated by the more frequent isolation of C. difficile from horses after penicillin treatment and experimental inoculation compared with nontreated horses.32 Age also apparently influences colonization rates. Baverud et al.16 reported subclinical colonization in 29% of foals less than 14 days of age, but in less than 1% of older foals.
Antimicrobial therapy is the main risk factor for development of CDAD in humans.8 Penicillins, cephalosporins, and clindamycin are considered the highest-risk antimicrobials in humans;33,34 recent evidence suggests that fluoroquinolone administration may also be an important risk factor.35 Antimicrobial therapy is presumably also an important risk factor for development of CDAD in horses. Initial studies linking antimicrobial therapy and CDAD in horses involved identification of colitis in mares whose foals were being treated with erythromycin. It was presumed that mares were being exposed to low levels of erythromycin during treatment of their foal.1 Subsequently, CDAD was reproduced experimentally with low-dose erythromycin administration, supporting the observational association of CDAD with erythromycin.36 Another study reported that all affected horses had been treated with beta-lactam antimicrobials;2 however, the risks of certain antimicrobials have not been objectively evaluated. The use of proton pump inhibitors (PPIs) is an important risk factor for CDAD in hospitalized humans.37 The relative risk of omeprazole administration in development of CDAD in horses has not been evaluated.
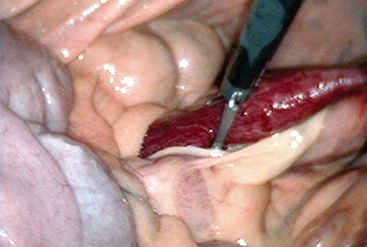
The role of clostridia in small intestinal disease of horses is less well understood, but recent evidence suggests that C. difficile may be a cause of duodenitis and proximal jejunitis (anterior enteritis; Fig. 44-4)38 (L. Arroyo, personal communication).
Clostridium perfringens
Unlike C. difficile, C. perfringens is a normal inhabitant of the equine intestinal tract and can be found in a large percentage of normal horses. Tillotson et al.39 isolated C. perfringens from 19% to 35% of broodmares and more than 90% of 3-day-old foals. Although C. perfringens is commonly found in healthy horses, it can be a pathogen. The pathogenesis of disease is unclear, and the reason that some horses develop disease likely relates to a combination of host and bacterial factors. A study evaluating foal management practices associated with C. perfringens diarrhea identified housing in a stall or drylot for the first 3 days of life; the presence of other livestock on the premises; delivery of dirt, sand, or gravel; and feeding of small amounts of hay and grain to the mare postpartum as risk factors for development of C. perfringens enteritis in foals.40 Antimicrobial treatment is likely a risk factor as well in adult horses and foals.
The type of C. perfringens is also a very important determinant of disease. Clostridium perfringens isolates are classified into different types based on production of four major toxins; alpha, beta, epsilon, and iota (see Table 44-1). In addition, a variety of other toxins may be produced, including beta-2 (β2) toxin and enterotoxin.7,41,42 While β2 toxin and enterotoxin are not classified as major toxins, they may be the most important toxins clinically and both have been independently associated with diarrhea in adult horses and foals.3,4,7,38,43 Type A is the predominant type in normal and diarrheic horses.39,43 All type A strains produce alpha toxin in varying amounts, although the role of alpha toxin alone in production of disease is questionable. While type A strains have been associated with disease in horses,44 this may be based on production of β2 toxin or enterotoxin by some strains,43 not because of inherent pathogenicity of all type A strains. A correlation between the presence of β2 toxin and fatal enterocolitis has been reported.42 Although less common, type C strains have been associated with enterocolitis horses, particularly severe disease in foals.23,39,45 There are limited reports of disease caused by type B46,47 and D strains.48
CLINICAL FINDINGS
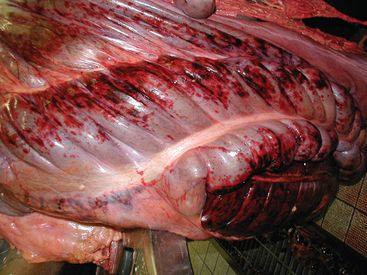
If the large colon is affected, diarrhea with varying degrees of depression, dehydration, toxemia, colic, anorexia, and pyrexia may be present. The clinical condition can be highly variable, ranging from mild diarrhea to peracute, rapidly fatal, necrohemorrhagic colitis (Fig. 44-5). In some horses, death may occur before diarrhea is passed.
If only the small intestine and cecum are affected, diarrhea may not be observed because of the tremendous absorptive capacity of the large colon. In these horses, colic, depression, pyrexia, hypoproteinemia, toxemia, and leukopenia may be present. If the proximal small intestine is involved, gastric reflux may develop.
Mortality rates are highly variable. With acute CDAD, mortality rates of up to 42% have been reported, with adult horses more likely to die than foals. Mortality of adult horses with acute CDAD is higher than for colitis of other etiologies.7 Similar data are not available for C. perfringens; however, certain strains of C. perfringens may be important causes of severe, fatal enterocolitis.42
DIAGNOSIS
Clostridium difficile
The “gold standard” for diagnosis of CDAD is detection of toxin B in feces using the cell cytotoxicity assay.49 However, this test is time-consuming, expensive, and not readily available commercially. Clinically, diagnosis typically requires identification of C. difficile toxin A or B or both in fecal samples.49,50 A variety of immunoassays are available and are widely used; however, none has been specifically validated for use in horses, and the sensitivity and specificity of the different tests are unclear. In human studies, sensitivities of different immunoassays range from 65% to 95%, with specificities between 70% and 100%.49,51 Care should be taken to determine which antigens an immunoassay is identifying. Some tests detect glutamate dehydrogenase
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree