CHAPTER 29 Embryo Transfer and Newer Assisted Reproductive Techniques for Horses
Embryo transfer has been the most widely utilized assisted reproductive technique for mares; it has been used to (1) obtain foals from performance mares, (2) obtain multiple foals from individual mares each year, (3) obtain foals from 2-year-old mares, (4) obtain foals from reproductively unsound mares, and (5) obtain foals from mares with nonreproductive health problems. In addition, embryo transfer has served as an important research tool for studying early pregnancy in the mare. Although embryo transfer was initially proposed as a promising method for obtaining foals from aged, subfertile mares, experiments utilizing oocyte transfer1 and embryo transfer2 have documented that many oocytes/embryos produced by aged, subfertile mares are inherently defective and have low survival rates after transfer to recipient mares; therefore, aged, subfertile mares are not optimal candidates for embryo transfer.
EMBRYO TRANSFER
The first successful equine embryo transfer was reported in 19723; however, it was not until the early 1980s that embryo transfer became an accepted clinical procedure in the equine breeding industry. At that time, widespread utilization of embryo transfer was limited by the need to maintain recipient mares at the site of embryo collection, or ship donor mares to a centralized embryo transfer facility. In the late 1980s, a technique for cooling equine embryos was identified4 and led to the development of a practical method of short-term (≤24 hours) storage and transportation of equine embryos. That development allowed embryos to be collected in the “field” and then shipped to a centralized facility for transfer to suitable recipient mares. The ability to transport cooled embryos provided veterinarians with the opportunity to offer embryo transfer service without the onerous task of maintaining recipient mares and eliminated the need to ship donor mares to a centralized facility.
Mare Management
Donor Mare
If indicated, a complete breeding soundness examination of the donor mare should be performed to assess her suitability for use in an embryo transfer program. If abnormalities are identified that warrant treatment (e.g., bacterial endometritis), appropriate therapy should be completed before embryo transfer procedures are performed. Breeding management of the donor involves teasing to monitor reproductive behavior; use of transrectal palpation and ultrasonography to monitor ovarian follicular activity and ovulation; and use of exogenous hormones to synchronize estrus and ovulation. When in heat, the donor is examined daily to monitor follicular growth, which allows optimal timing of insemination with fresh, cooled, or frozen semen. The day of ovulation is detected is designated as Day 0. Historically, a practical and efficacious means of superovulating mares has not been available; however, a commercially available formulation of equine follicle stimulating hormone (eFSH) has recently become available. Mares treated with eFSH had an average of 3.9 ovulations and 1.9 embryos recovered during a treatment cycle compared to 1.0 ovulation and a 0.5 embryo recovery rate for control mares.5 Work is continuing to develop an optimal eFSH treatment protocol for superovulation in mares.
Recipient Mares
Proper selection and management of recipient mares may be the most important factor affecting the success of an embryo transfer program. Recipient mares should have normal estrous cycles, and must be free of uterine or ovarian abnormalities. The optimal age of recipient mares is 3 to 10 years. Synchronizing estrus between donor and recipient mares can be accomplished with routine protocols using prostaglandin F2α (PGF2α) alone or in combination with exogenous progesterone.6 When in heat, recipients are examined daily with transrectal palpation and ultrasonography to monitor follicular growth and detect ovulation. The “window” of synchrony between ovulation in recipient and donor mares is +1 to −3 days (i.e., the recipient mare can ovulate 1 day before to 3 days after the donor mare).7 In an effort to eliminate the need for synchronizing recipient and donor mares, progestin-treated, ovariectomized mares have been used as embryo recipients8–11; however, the success with their use has been variable, and the practice has not been widely adopted.
Embryo Recovery
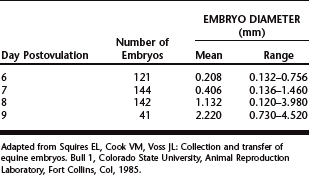
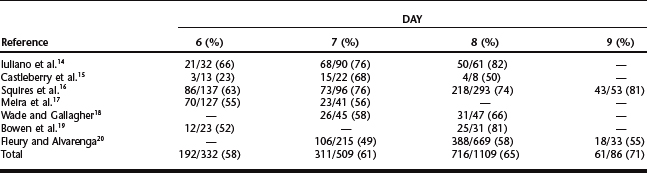
Equine embryos are selectively transported through the oviduct into the uterus between days 5 and 6 after ovulation,12 at which time they are at the compact morula to early blastocyst stage of development. After entering the uterine lumen, the size of the embryo increases dramatically as it develops into an expanded blastocyst (Table 29-1).13 Although embryos can be recovered on days 6 to 9 (Table 29-2),14–20 the optimal time of embryo collection is day 7 or 8; currently, our preference is to perform embryo recovery on day 8. The primary indication for recovering embryos on day 6 is for freezing embryos.7 Embryos are not routinely collected on day 9, because their transfer success rate is generally lower than day 7 or 8 embryos.7
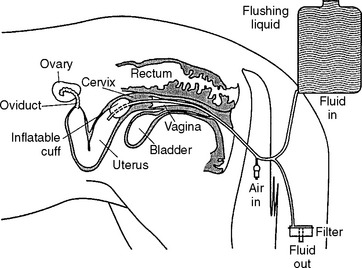
Embryo collection is performed using transcervical uterine lavage (Fig. 29-1). We currently use an 80-cm silicone balloon-tipped catheter (VEUF-80, Bivona, Inc., Gary, IN 46406) with an inside diameter of 8.0 mm (French size 33); however, other styles of flushing catheters are available. After placing the catheter, the uterus is flushed three to four times with warm (30° to 35° C) flush medium. Historically, the most widely used flush medium has been Dulbecco’s phosphate buffered saline (DPBS) containing 1% (v/v) fetal or newborn calf serum, penicillin (100 units/mI), and streptomycin (100 μg/ml); however, more recently, many practitioners have begun to use a zwitterion-buffered flush medium containing antibiotics and bovine serum albumin (emCare Complete Flush Solution, Professional Embryo Transfer Supply, Inc., Canton, TX 75103-0188) or polyvinyl alcohol (ViGro Complete Flush Solution, AB Technology, Pullman, WA 99163).
Regardless of which medium is used, using gravity flow, the uterus is filled with 1 to 2 L of medium during each flush (4 to 8 L used during entire procedure). After filling the uterus, the fluid is allowed to flow back out through the catheter and is passed through a 0.75-μ embryo filter. It is important that the embryo filter not overflow or run dry; filters are available that are designed to prevent both from occurring. The fluid passing through the filter is collected to monitor its recovery. After the first flush, the uterus is massaged per rectum during subsequent flushes, which may aid suspension of the embryo(s) in the medium and enhance fluid recovery. The majority (>90%) of fluid infused into the uterus should be recovered, and should be free of cellular debris or blood. Recovery of cloudy fluid indicates the mare had an active endometritis at the time of the embryo recovery and warrants further diagnostic evaluation. When present, blood contamination is often associated with vigorous massage of the uterus or manipulation of the catheter.
At the completion of the flush, the filter cup is emptied into a sterile search dish with grid and the filter is rinsed with approximately 50 ml of flush medium. The fluid is then examined for the embryo(s) using a stereo-microscope at approximately 15× magnification. Larger embryos (e.g., day 8) are generally visible with the naked eye. When an embryo is identified, it is washed by transferring it sequentially through several (3 to 10) 1-ml drops of “holding medium,” which consists of an enriched formulation of flush medium; after washing, the embryo is placed into a small petri dish containing the same medium. The embryo is then examined at high magnification (40 to 80×) and graded on a scale of 1 (excellent) to 4 (poor).21 Embryos can be handled using a 0.25- or 0.5-ml semen-freezing straw, 25-μl glass capillary pipette, or other suitable instrument attached to an appropriate syringe. Whenever an embryo is drawn into a handling instrument, the medium containing the embryo should be surrounded on each side by an air bubble and blank medium. This prevents the embryo from accidentally being pulled out of the instrument should the tip touch something absorbent.
Once embryos are placed into the holding medium, they should either be expeditiously processed and packaged for transport (or frozen) or be transferred into an appropriate recipient mare.22 While awaiting packaging for transport or immediate transfer to a recipient, equine embryos appear to be quite tolerant of temperatures between room temperature (25° C) and body temperature (37° C). However, efforts should be made to prevent rapid or extreme changes in temperature.
Packaging Embryos for Transport
Since the late 1980s, equine embryos have been cooled and transported using methods developed by Carnevale and associates,4 which utilizes Ham’s F-10 nutrient mixture as the holding/cooling medium. Prior to use, Ham’s F-10 medium must be buffered by diffusing a mixture of 90% N2, 5% O2, and 5% CO2 gas through the medium for 3 to 5 minutes, after which it is supplemented with 10% (v/v) fetal or newborn calf serum, penicillin (100 units/ml), and streptomycin (100 μg/ml). Because Ham’s F-10 medium must be “gassed” prior to use, it requires having an appropriate compressed gas cylinder and regulator. In addition, once gassed, Ham’s F-10 has a limited shelf-life; therefore, many practitioners choose to have the embryo transfer facility that will receive the embryo provide Ham’s F-10 just before the embryo collection as part of an embryo shipping kit. More recently, equine embryos have been successfully cooled and transported using the complete medium holding solutions described above,22 which eliminates the need to prepare the special Ham’s F-10 solution.
Freezing Embryos
The primary advantage of freezing embryos is that it obviates the need to have a synchronized recipient mare available at the time of embryo collection, since the embryo can be frozen and then transferred at a later date when a recipient mare is available. In addition, it would facilitate the international movement of equine embryos. Although “conventional” embryo freezing procedures using glycerol or other cryoprotective agents have been reasonably successful for equine embryos, to do so requires expensive and specialized equipment, as well as approximately 90 minutes to complete the freezing procedure. In addition, after thawing, embryos are generally moved through a series of solutions to dilute the cryoprotectant and other components of the freezing medium before transfer, which makes the post-thaw process laborious. In contrast to conventional freezing procedures, vitrification is a process that utilizes high concentrations of cryoprotectants that can be completed rapidly (<15 min) without specialized equipment using a commercially available vitrification kit.23 After thawing, vitrified embryos can be directly transferred from the straw into the recipient mare, since further dilution or handling of the embryo is not necessary.
To vitrify an embryo, after “washing” the embryo with a standard embryo holding medium, the embryo is moved sequentially through three separate vitrification solutions (VS1, VS2, and VS3), and then it is loaded into a 0.25 ml polyvinyl chloride straw.23 The embryo is held in VS1 for 5 minutes and then transferred to VS2 for an additional 5 minutes, after which it is transferred to VS3. The embryo should remain in VS3 for less than 1 minute, which includes the time to load the embryo into the straw and initiate freezing. The embryo is loaded into the straw in approximately 30 ul of VS3; the embryo (in VS3) is “sandwiched” in the straw between an air bubble on either side and approximately 90 ul of a 0.5 M galactose solution beyond that (on both sides of the embryo). The galactose solution will be mixed with the embryo in VS3 after thawing. Once the straw is loaded, the open end is sealed, and the straw is placed into liquid nitrogen vapor for 1 minute before it is plunged into the liquid nitrogen, after which it is moved to a storage tank. A straw is thawed by holding it in room temperature air for 10 seconds, and then it is placed into a room temperature (20° to 22° C) water bath for an additional 10 seconds, after which the contents of the straw are mixed by “flicking” it five to seven times (like a thermometer). The straw is then allowed to lay flat for 6 to 8 minutes at room temperature, after which the tip of the straw is opened and then loaded into a standard transfer gun for transfer into a suitable recipient mare. Using this vitrification technique, Carnevale et al.23 reported a 62% (16/26) pregnancy rate at day 16 after transfer of embryos. However, like conventional freezing procedures, only small (<300 micron) embryos tolerate vitrification procedures suitably. Therefore, embryos that will be vitrified should be collected on day 6 to very early on day 7.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree