CHAPTER 123 Embryo Technologies in South American Camelids
The application of reproductive biotechnology is in the formative stages for the domestic species of South American camelids (llama and alpaca) and is virtually nonexistent for the wild species (guanaco and vicuña). Llamas and alpacas represent a valuable economic and biologic resource for Peruvian, Bolivian, and Chilean people living in the high plains of the Andes,1 and have become a consistent feature of North American livestock production. World production of fiber from alpacas exceeds 4 million kilograms, worth more than $12 million ($US).2 Peru has over 3 million alpacas and 1 million llamas, Bolivia has more llamas (2 million) than alpacas (325,000), and Chile has the smallest number of animals (33,000 alpacas and 67,000 llamas). There are more than 150,000 registered llamas and alpacas in North America.
OVARIAN SUPERSTIMULATION
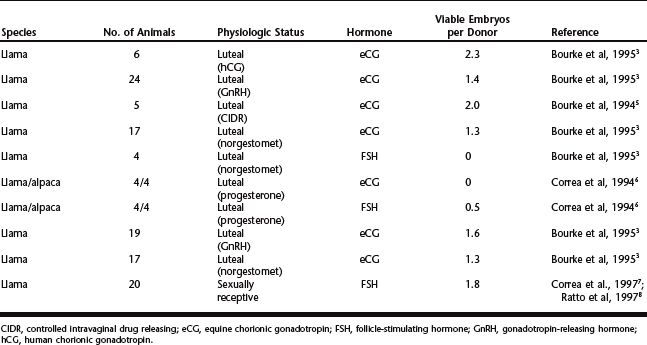
Results of studies on ovarian superstimulation and embryo production in llamas and alpacas are summarized in Table 123-1. Superstimulation has been attempted using equine chorionic gonadotropin (eCG) and follicle-stimulating hormone (FSH) during a luteal phase (induced by eliciting ovulation or by progestogen treatment) or during the sexually receptive phase. After superstimulatory treatment, the females were mated and given gonadotropin-releasing hormone (GnRH) or human chorionic gonadotropin (hCG) to induce ovulation. Superstimulatory treatment schemes may be summarized as follows:
The preoccupation with inducing a luteal phase before or during superstimulation in camelids is enigmatic, but may simply reflect an empirical bias to conventional methods used in other ruminants. The number of ovulations or corpora lutea (CL) varies widely among studies, ranging from 2 to more than 11 per animal. Much of the variation may be attributed to the variation in follicular status at the time superstimulatory treatments were initiated. Presumably, follicular dominance will suppress the superstimulatory response in llamas and alpacas, as it does in cattle, but this remains to be tested. In a recent study9 comparing the efficacy of FSH and eCG, treatments were initiated at the time of wave emergence and both hormones effectively induced ovarian superstimulation. FSH treatment induced the growth of 18 follicles (≥6 mm) per animal, on average, and eCG induced the growth of 17.
EMBRYO COLLECTION AND TRANSFER
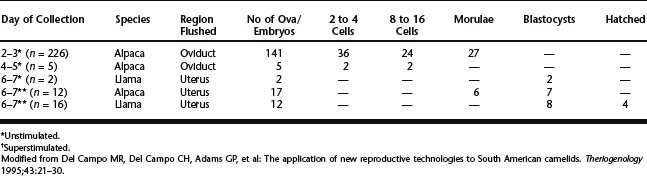
Researchers from Peru reported the first collection of zygotes from the oviducts of alpacas after spontaneous ovulation and from superovulated females by laparotomy 3 days after ovulation.10 The flushes were done normograde, from the ovarian end of the oviduct to the uterine end; authors suggested that the muscular uterotubal junction made retrograde flush impossible.11 Embryos have been collected on various days after breeding, using surgical or nonsurgical techniques in unstimulated alpacas and guanacos and superstimulated alpacas and llamas (Table 123-2).
Table 123-2 Embryo Collection on Different Days After Breeding from Unstimulated and Superstimulated Llamas and Alpacas
The nonsurgical method of embryo collection is similar to that used in cattle and consists of the introduction of a catheter through the cervical canal and placement of the cuff just cranial to the internal cervical os. Both uterine horns are flushed simultaneously by infusing collection medium until the horns are distended and then the medium is collected by aspiration or gravity flow. The process is repeated several times until 500 to 1000 ml of medium are recovered.4,12–15 Uterine flushing has been done on days 6.5 to 12 after mating, but embryo recovery has been frustratingly variable. Generally less than 50% of the zygotes have been recovered, based on CL counts, regardless of the method of embryo collection.12 The recovery of embryos by surgical flushing of the oviduct and uterus 7 days after mating in 20 llamas treated with pFSH is summarized in Table 123-3. Llamas were mated either 0 hours or 36 hours after the last pFSH treatment.16
Table 123-3 Ovarian Response (Mean ± SD) and Ova/Embryo Collection Rate after Flushing the Oviduct and Uterus in Llamas Mated 0 Hours or 36 Hours after the Last FSH Administration*
| End Point | Mating at 0 Hours | Mating at 36 Hours |
|---|---|---|
| Number of llamas | 10 | 10 |
| Number of corpora lutea | 4.5 ± 4.2 | 13.8 ± 8.4 |
| Number of follicles ≥ 8 mm | 6.5 ± 5.4 | 7.5 ± 8.3 |
| Embryo collection rate | 27/45† (60%) | 27/138† (20%) |
| Embryo collection from uterus | 17/27 (63%) | 16/27 (60%) |
| Embryo collection from oviduct | 10/27 (37%) | 11/27 (40%) |
| Number of blastocysts from uterus | 17 | 16 |
| Number of blastocysts from oviduct | 3 | 0 |
| Number of unfertilized oocytes | 7 | 11 |
* Given every 12 hours for 5 days, total dose of 200 mg Folltropin.
† Total number of corpora lutea.
From Ratto MH: Induction of superovulation in llamas. Master’s thesis, Universidad Austral de Chile, Valdivia, Chile, 1995.
Embryo Transfer
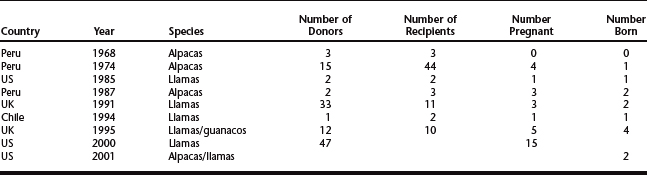
Over the past 30 years, approximately 13 crias have been born throughout the world as a result of embryo transfer techniques12 (Table 123-4). The first birth of an alpaca using surgical collection and transfer techniques was reported in 1974.11 Of 44 recipients, 4 became pregnant, 3 aborted, and 1 gave birth. The first llama born using nonsurgical collection and transfer was reported in a study done in North America17 in which collection and transfer were done 7 days after GnRH treatment. In 1987, the birth of 2 alpacas was reported in Peru through the use of nonsurgical collection and transfer.18 Six live cria were born in the United Kingdom during 1992 to 1995, from 27 embryos transferred nonsurgically to 21 synchronized recipients.4,5 Interestingly, only recipients synchronized with GnRH became pregnant; no pregnancies resulted in those that received progestagen implants. In Chile, the birth of 1 llama cria after 2 nonsurgical embryo transfers was reported in 1994.19 More recently, Canadian scientists reported the recovery of 23 embryos from 5 superstimulated llamas,20 and an American study reported the recovery of 37 embryos from 47 unstimulated donors (79%), 41% of which established pregnancies after transfer to recipients.21 The first report of successful interspecies transfer in camelids appeared in 2001 after 2 alpaca crias were born to llama recipients.22
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree