Fig. 1.
Instruments for brain slice preparation. (a) Dissecting tools for brain slice preparation. (b) A Vibratome (Leica VT1200) has been set up for slicing.
3.
A water bath (preheated to 32°C) holding a submerge chamber for slice incubation (Fig. 2d).
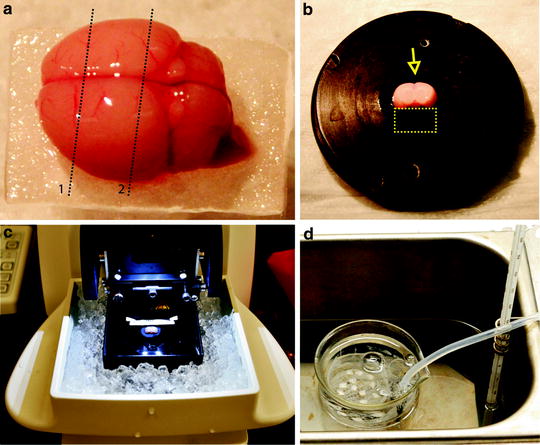

Fig. 2.
Cutting neocortical slices. After the brain is removed from the skull and placed on a piece of filter paper two parallel coronal cuts are made as indicated by the dotted lines (a). The brain is then glued onto the slicing stage, with the caudal surface on bottom (cutting surface of line 2) and the dorsal side facing the blade (arrow). When a brain from a young rat is cut, a small block of agar (dotted line rectangular) should be glued onto the stage to support the brain (b). The brain is cut with a Vibratome (c), and slices are transferred to an incubation chamber maintained in an incubator at 32°C for 1 h (d).
2.2 Instruments for Field Potential Recording
1.
Sutter P-97 micropipette puller (Fig. 3a, Sutter Instruments).
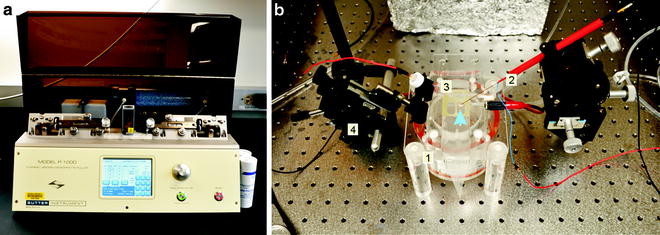

Fig. 3.
Field potential recording setup. (a) A pipette puller is used to make recording electrode from glass capillaries. (b) On top of an interface chamber (1) is a cortical slice (arrow), which is in contact with a stimulating electrode (2) and a recording glass electrode (3). Both of them are attached to micromanipulators (4).
2.
Stereo microscopy (American Scope).
3.
DP 304 Differential amplifier (Warner Instruments).
4.
Axon Digidata 1440 A/D–D/A board (Molecular Devices).
5.
Analog stimulation isolator (Model 2200, A&M systems).
8.
A gravity-fed perfusion system.
9.
Computer with software for data acquisition and analysis (e.g., pClamp from Molecular Devices).
2.3 Solutions
1.
Cutting solution contains the following three groups of ingredients (in mM):
(a)
252 sucrose, 10 glucose.
(b)
3 KCl, 0.2 CaCl2, 6 MgSO4, 1.3 NaH2PO4.
(c)
26 NaHCO3.
2.
Artificial cerebrospinal fluid (ACSF) contains the following two groups of ingredients (in mM):
(d)
124 NaCl, 2 KCl, 2 CaCl2, 1 MgSO4, 1.3 NaH2PO4, 10 dextrose.
(e)
26 NaHCO3.
For storage, stock solutions (a–e) at 10× concentration should be individually prepared and stored in refrigerator at 4°C. Final solution is made by mixing together equal amount of each solution (a–c solutions for cutting solution, d and e solutions for ACSF), and diluted to final concentration before use. The cutting solution is then ice-colded, and is bubbled with 95% O2 and 5% CO2.
2.4 Stimulating and Recording Electrodes
3 Procedures
3.1 Preparation of Cortical and Hippocampal Slices
3.1.1 Neocortical Slices’ Preparation
1.
A rat is anesthetized with 50 mg/kg pentobarbital sodium i.p.
2.
After decapitation, the brain is rapidly removed with a spatula and submerged in ice-cold cutting solution for 1 min.
3.
Place the brain on a small piece of filter paper with ventral side on bottom; make two parallel coronal cuts with one slightly anterior to the cerebellum and the other at about one-third of cortex length from the olfactory bulb (Fig. 2a).
4.
The brain is glued onto the cutting stage of a Vibratome with instant glue, with the caudal surface on the bottom and dorsal surface facing the blade (Fig. 2b). A cut along the midline may be made carefully so that the two hemispheres can be separated. A block of 4% agar should be glued on the ventral side of the brain, which provides support to the brain on the opposite side against the direction of blade movement (Fig. 2b).
5.
The brain is submerged into ice-cold ACSF together with the cutting stage. Coronal cortical slices (400-μm thick) are cut using a Vibratome (Fig. 2c).
6.
Use a large-mouth Pasteur pipette to transfer slices into an incubation chamber, which is filled with ACSF preheated to 32°C and constantly bubbled with 95% O2 and 5% CO2 (Fig. 2d). The incubation chamber is maintained at 32°C for 1 h in an incubator, and then moved to room temperature. Now the slices are ready for use.
3.1.2 Hippocampal Slice Preparation
The general procedures are similar to neocortical brain slice preparation, expect that sagittal brain slices are cut, and then whether the nonhippocampal regions are trimmed or not depends on the purpose of experiment. It is also a common practice that hippocampus is first dissected out, and then transverse hippocampal slices are cut with a tissue chopper.
1.
Remove the brain with a spatula, place it on a small piece of filter paper with ventral side on bottom, and then make two parallel coronal cuts similar to what is shown in Fig. 2a.
2.




Cut the brain in half through midline, and then glue hemispheres onto cutting stage, with the medial side on bottom and posterior side facing Vibratome blade.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


