Fig. 1.
Photograph of major components of a typical electrophysiology station.
Anti-vibration table. This is to eliminate vibration noises that would be detrimental to patch clamp. The table is supported by pressured air that is supplied by an air tank. The table will hold the microscope and micromanipulators.
Faraday cage. The Faraday cage shields out the sensitive patch-clamp signals from unwanted electrical noise.
Microscope. The type depends on the type of experiments either an upright or an inverted microscope can be used for patch-clamping. Generally, an inverted microscope is good for cultured or acutely dissociated cells. In contrast, an upright microscope is necessary for acute or cultured brain slices. Currently, all the major manufactures sell upright microscopes specifically designed for patch-clamping on slices. The special upright microscopes are equipped with a fixed stage and long working distance water immersion objectives (20× or 40×). Unlike the traditional upright microscope where the stage moves for focusing, in these microscopes the objective moves for focusing so that the experimenter can change the view of the slice without disrupting an already established patch recording. To visualize the cells in the brain slices, the microscope needs to be equipped with appropriate optics, such as differential interference contrast (DIC) combined with an infrared charge-coupled device (CCD) camera. For experiments using patch-clamping combined with fluorescence ion imaging, such as Ca2+ imaging, the microscope needs to be equipped with fluorescence components, such as filter systems, shutter control and appropriate light source.
Amplifier and data acquisition software. The electric currents or voltage changes generated by ion channels and receptors are usually small, and need to be amplified. Several major manufactures make amplifiers used for patch-clamping. They included but are not limited to Molecular Devices (originally known as Axon Instruments, Silicon Valley, CA, USA), HEKA Electronik (Lambrecht/Pfalz, Germany) and many other companies. The newest versions of patch-clamp amplifiers are knob-less computer-controlled devices, which are easier to use. The amplifiers usually come with a data acquisition software package, which is used to control all operations of the patch-clamping, data acquisition and storage and offline data analysis following the acquisition.
Micromanipulators. To place patch pipettes on cells, it is very important to be able to precisely control the movement of the pipette. There are many types of commercially available micromanipulators, including mechanical, hydraulic and motorized ones. Motorized micromanipulator tend to be more stable and precise, but more expensive as well. The manipulators usually are mounted on the fixed stage of the microscope. At least one manipulator is needed to hold the headstage for patch-clamping, which is the electronic probe connected to the amplifier and holding the patch pipette. If electrical stimulation, for example, for studying the neurotransmission of neurons in a brain slice, a second micromanipulator is needed to hold the stimulating electrode from the electrical stimulator.
Electrical stimulator: Stimulators may be analogue, digital with knobs or knob-less computer-controlled digital stimulators. The latter is favourable because of its capacity to integrate into the data acquisition program.
Pipette puller and microforge. To precisely control the size and shape of glass micropipette used for patch-clamping, a multiple step programmable horizontal puller (such as the Flaming/Brown micropipette puller P-97, Sutter Instrument) is helpful and has some advantages over the simple two stage vertical pullers. To achieve a better seal of the pipette tip over the cell membrane, a microforge is needed to polish and smooth the tip of the pipettes.
Vibratome. In studies using acutely isolated brain slices, a vibratome is needed to produce viable slices. There are two major vibratome makers, namely Leica Microsystems (Wetzlar, Germany) and The Vibratome Company (Bannockburn, IL).
2.2 Materials Used for Patch-Clamping
Grounding electrode. This is usually made from a silver wire and connected to the grounding outlet of the headstage through a pin. The silver wire should be chloride-coated by simply using household bleach. Alternatively, the silver wire can be connected to an agar bridge, which is made from a U-shaped capillary tube filled with agar that is made with the bath solution.
Glass capillaries. Capillaries are used to make the electrodes for patching on cells. Different types of glass can be used. The key is to choose glass that produces the least noise or at least less than that of the electrode. In this regard, borosilicate and aluminosilicate glass are the most commonly used. Quartz glass has the lowest noise, but it is more expensive and requires a special laser puller to produce the electrodes. It can be used for extreme low noise recording, such as single channel recordings.
Buffer solutions. In general, the bath solution for isolated cells is composed of a solution designed to mimic the extracellular solution in vivo, which is typically high in sodium (Na+). Conversely, the solution used to fill the patch pipette (recording electrode) is composed to mimic the intracellular contents, which has a high concentration of K+. For example, the bath solution used in our laboratory contains (in mM): NaCl 100, KCl 50, MgCl2 1, CaCl2 2, HEPES 10, and glucose 10, pH 7.4. The internal (patch pipette) solution consists of (in mM): KCl 142.6, KOH 2.4, MgCl2 2.5, BAPTA 10, and HEPES 10, pH 7.2 (7). In addition, these solutions could be modified according to experimental purposes depending on what ionic currents one is trying to record. For example, BAPTA was included in the pipette solution to buffer intracellular Ca2+ to completely block large conductance, Ca2+-activated K+ channel currents in the study of inward rectifying K+ currents (7). There are numerous other variations in terms of solutions and also of the configuration of the patch-clamp system. In some studies, 200 mg/ml of nystatin was added to perform nystatin-perforated patch technique to measure the resting membrane potential in the current-clamp mode (I = 0) (7). Drugs to block some channels may be used in order to isolate currents due to other channels (1).
3 Procedures
3.1 Dissociation of Smooth Muscle Cells from Arteries
The smooth muscle cells from cerebral arteries of dogs (1–4, 6, 7), rabbits (5, 8, 9), rats (13–15) and humans (9) have been used so far for studying vasospasm after SAH (Fig. 2). Smooth muscle cells usually are isolated by cutting the artery into small pieces and incubating the pieces for 30 min in enzymes (500 U/ml collagenase type IV, 50 U/ml elastase, 100 U/ml DNase I and 1 mg/ml trypsin inhibitor). Smooth muscle cells are then separated by gentle trituration while suspended in a buffer solution (dissection solution + 0.2% bovine serum albumin), stored at 4EC, and used within 12 h of isolation. The identity and health of isolated smooth muscle cells can be confirmed by positively stained for α-actin (a marker for smooth muscle cells) and contraction in response to agonists (KCl, 60 mM or serotonin, 1 mM).
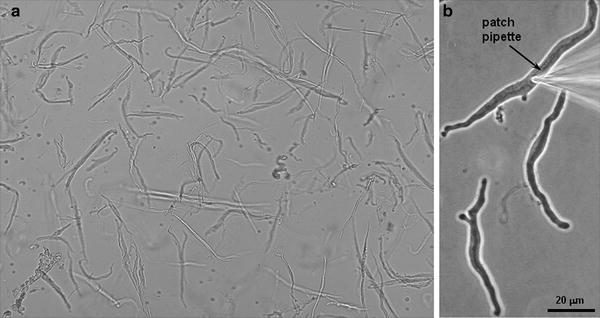

Fig. 2.
(a) Acutely dissociated smooth muscle cells from normal dog basilar artery. (b) Normal dog basilar artery smooth muscle cell with patch pipette advanced onto the cell membrane.
3.2 Whole Cell Patch Clamp of Smooth Muscle Cells
Smooth muscle cells are allowed to adhere to a glass coverslip mounted at the bottom of the recording chamber. Borosilicate glass pipettes with resistances of 2–5 mΩ are advanced with the micromanipulator slowly onto the membrane of the cell to be patched. One then applies gentle suction to the pipette through tubing attached to the side port of the pipette holder. This forms a gigaseal with the cell membrane [the seal resistance usually should be more than 109 Ω (gigaseal)). Next, the cell membrane of the cell is ruptured by applying a sudden stronger suction to achieve the whole-cell configuration.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


