Ingredients
200 Flasks
100 Flasks
50 Flasks
25 Flasks
10 Flasks
100 Vials
Water
Agar
Karo
8400 ml
90 g
1200 ml
4200 ml
45 g
600 ml
2100 ml
23 g
300 ml
1050 ml
12 g
150 ml
420 ml
5 g
60 ml
1470 ml
17g
210 ml
Bring water, agar, and Karo to a boil. Insure that the agar is completely dissolved
Corn meal
Brewer’s yeast b
Water
980 g
130 g
2900 ml
490 g
65 g
1450 ml
245 g
33 g
725 ml
17g
17g
363 ml
49 g
7 g
145 ml
180 g
24 g
500 ml
Add corn meal, yeast and water. Bring to a rolling boil to cook the corn meal
Propionic acid
68 ml
34 ml
17 ml
9 ml
3 ml
12 ml
Add propionic acid when boiling is complete
The first set of ingredients, corn syrup, agar, and water, should be brought to a boil to ensure that the agar is completely dissolved. If molasses is used, it must be sulfur free. Care should be taken that the food is not overcooked. After the addition of the cornmeal and yeast, the food should be brought to a rolling boil and then removed from the heat. It should be allowed to stand until the boiling is over before adding propionic acid. If acid is added too soon, it will boil away and the food will become moldy. If too much propionic acid is used, the flies will die. It is important to use dead yeast in the food preparation and live yeast before placing flies on the food. Do not over yeast the food because the flies will not lay eggs. If desired, a moderate solution of yeast in water can be used instead of yeast grains. In this case, use only one or two drops of the suspension in each vial immediately before adding flies.
2.3 Immunoblot Assays
For α-synuclein dot blot assays, 1,000 heads were removed from CO2 anesthetized 14-day-old w 1118 and transgenic α-synuclein (UAS) flies on glass plates at 4°C. The heads were transferred into Eppendorf tubes on ice, homogenized in 10 mM PMSF and then centrifuged at 13,000 rpm for 15 min at 4°C. The supernatants were then blotted onto nitrocellulose paper, which was subsequently immersed in blocking solution for 16 h. This solution was discarded and the membrane was washed in TTBS for 15 min. Primary antibody (mouse anti-synuclein; Zymed) diluted in buffer was incubated with the membrane for 90 min followed by secondary anti-mouse antibody. The secondary antibody was decanted and the membrane was washed with TTBS for 15 min. The coloring agent, BCIP/TNBT, was added for 10 min and the membranes were then washed in water for 5 min to stop the reaction.
2.4 Western Blot Alpha-Synuclein Assays
Transgenic α-synuclein (UAS) and w 1118 flies were anesthetized in CO2, placed in plastic vials, and quick frozen in a dry ice-ethanol bath. The frozen flies were placed on tiles that had been kept in a freezer and heads were collected from 400 flies of each genotype. Heads were then separately homogenized in 40 μL of PMSF and 100 μL of lysis buffer (Cell Signaling) containing protease inhibitor. It is important that the grinding of heads and the homogenizing process take place in an ice bath to prevent denaturation of proteins. The grinding process must be vigorous because of the very small size of the brain cells. The heads were homogenized, freeze–thawed, and sonicated three times creating three extracts which were numbered in consecutive order (see Fig. 1, top). After centrifugation, 32 μL of each extract supernatant was added to 8 μL of sample (loading) buffer (Sigma) containing beta-mercaptoethanol and Laemmli buffer. Into another Eppendorf tube 1 μg/μL of synthetic human α-synuclein (Sigma) was placed in 9 μL of sample buffer. The samples were heated for 10 min at 95°C and then subjected to gel electrophoresis at 145 V for 90 min. The gels were then transferred onto a nitrocellulose membrane at 25 V for 30 min and then incubated in 5% condensed milk solution for 60 min at 5°C. After washing for 30 min, the membrane was incubated with primary mouse antibody diluted in TBST (1:1,000) at 5°C for 16 h. After washing for 30 min, the membrane was incubated with secondary antibody diluted with TBST (1:1,000) at 5°C. The membrane was washed again and stained with BCIP/NBT overnight, washed, and blow dried. A protein ladder (Bio-Rad) was also run on each membrane.
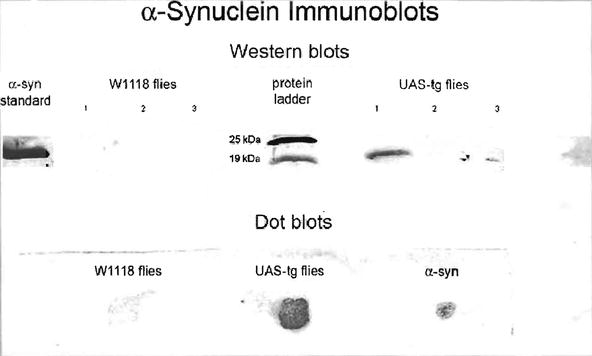





Fig. 1.
Western blots (top) in the absence of GAL4 drivers show the presence of α-synuclein in the heads of homozygous UAS-transgenic flies. The head samples in the western protocol were from three successive extracts of 400 heads. All the α-synuclein proteins were found in the initial extract. The dot blot samples (bottom) were extracted once from 1,000 heads each and transferred to a nitrocellulose membrane.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


