Fig. 1.
Electrophysiological equipment for EEG, EP, and extracellular single-unit recordings. (a) Representative of a digital EEG Comet XL Lab-based PSG system from GRASS Technologies. (b) Representative of an electrophysiological recording system from AxoClamp 2B from Axon Instrument (Fig. 1b-1) with HS-2A headstage (Gain: ×1 LU) for evoked potential recordings. (c) Representative of an electrophysiological recording system for extracellular single-unit recordings.
EP. An AxoClamp 2B amplifier from Axon Instrument (Fig. 1b-1) with HS-2A headstage (Gain: ×1 LU) is used to study slow-pain responses by selective stimulation of peripheral C-fibers.
Single-unit recording. We use A-M System (Model 1800, Fig. 1c-1) for single-unit recording.
2.1.2 Oscilloscope
An oscilloscope is used to constantly monitor the electrical signals detected by the recording electrode and amplified by the amplifier for the extracellular single-unit recordings from midbrain dopaminergic neuron. The oscilloscope is used to monitor the action potential waveform which permits us to differentiate between dopaminergic and nondopaminergic neuron firing. Our oscilloscope is manufactured by Hitachi (VC-6524, Fig. 1c-3). In addition, an audio system is used to monitor membrane potential changes caused by action potential firing (Fig. 1c-5).
2.1.3 Stimulator
We use A-M System isolated pulse stimulator (Model 2100) for EP study (Fig. 1b-3). It can be used in either voltage- or current-stimulation modes.
2.1.4 Computer and A-D Converter
A computer is used for both data acquisition during experiments and data analysis thereafter (Fig. 1a, b-7, c-6). An analog-digital converter is used so that signals can be recognized by the computer. It is also used in reverse to send command signals from the computer to the amplifier. We use Digidata 1322A (from Axon Instrument) for EP recording (Fig. 1b-4) and Sky-A4 Bioelectric signal processing system (Fudan University; Shanghai, China) for single-unit recording (Fig. 1c-2).
2.1.5 Data Acquisition and Analysis Software
For EP experiments, we use pClampex 9.2 for data acquisition and pClampfit 9.2 for data analysis for our PC. For single-unit recording, we use software package from Sky-A4 Bioelectric signal processing system Mathlib (Fudan University; Shanghai, China).
2.2 Other Equipment
2.2.1 Vibration Isolated Table and Faraday Cage
2.2.2 Electrode Puller
We use PP-830 vertical electrode puller (Narashige, Japan).
2.2.3 Stereotaxic Frame
We use Model SR-6M (Narashige, Japan, Fig. 2-1).
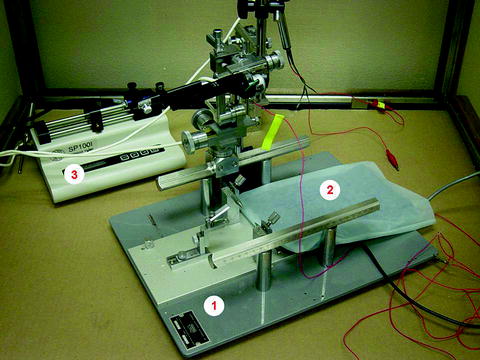

Fig. 2.
Representative of an atereotaxic frame (Model SR-6 M, Narashige, Japan, Fig. 2-1), a homeothermic blanket control unit and heating pad from Harvard Apparatus (Fig. 2-2), and an automatic syringe pump from Harvard Apparatus (Fig. 2-3).
2.2.5 Heater
Our homeothermic blanket control unit and heating pad are from Harvard Apparatus (Fig. 2-2).
2.3 Anesthesia
EEG. (Rodents): 87 mg/kg ketamine plus 13 mg/kg xylazine (i.p.)
EP. (Cat): α-chloralose 60 mg/kg (i.v.)
Single-unit recording. (Rodents): Chloral hydrate (400 mg/kg, i.p.)
3 Procedures
3.1 Preparing Electrodes
3.1.1 Recording Electrodes
EEG recording electrode. Epidural recording electrodes made from # 0–80 × 1/8-inch stainless steel screws were placed at the following stereotaxic coordinates (Paxinos and Watson, 1980): A-P +2.0 mm, lateral ±3.0 mm and A-P −4.0 mm, lateral ±3.0 mm. Electrodes were attached to a small plastic jack box, which is connected to the EEG machine.
EP recording electrode. EP recording electrodes can be made from either glass pipettes (containing 2 M NaCl ) or final metal wire. In our laboratory, we use a silver-ball recording electrode to measure EP from the surface of the cerebellar cortex.
Single-unit recording electrode. Glass microelectrodes (5–10 MΩ) are filled with 2 M NaCl solution containing 2% pontamine sky blue dye.
3.1.2 Stimulating Electrodes
A bipolar tungsten stimulation electrode (World Precision Instruments, Sarasota, FL) or a bipolar platinum wire (diameter 0.005″ insulated by Teflon, A-M System Inc.) stimulus electrode (house made) is used in our laboratory for EP recordings.
3.1.3 Reference Electrodes
Silver wire (0.025″, A-M System) coated with silver chloride is used as reference electrode for EP and single-unit recordings. The reference electrode is wrapped with cotton soaked with 0.9% sodium chloride and placed subcutaneously near the open skull. The reference electrode is connected to the ground by taping it tightly onto the ear bar of the stereotaxic frame.
3.2 Animal Preparations
3.2.1 EEG Recordings from a Status Epilepticus Animal Model
We used the lithium/pilocarpine model in 10-week-old male Sprague Dawley rats (approximately 160 g from Charles River Laboratories) to induce SE following the procedure described by Walton and Treiman (10). Rats were housed singly before surgery with food and water available ad libitum with a 24-h diurnal light cycle (lights on from 0700 to 1900 daily). At the time of electrode placement, rats were anesthetized with 87 mg/kg ketamine plus 13 mg/kg xylazine delivered by intraperitoneal injection (IP). Epidural recording electrodes were placed at the following stereotaxic coordinates (11): A-P +2.0 mm, lateral ±3.0 mm and A-P −4.0 mm, lateral ±3.0 mm. The electrodes were attached to a small plastic jack box, secured with dental acrylic, and the wound was sutured. The animals were allowed to recover for at least 24 h before further handling and given 1 week to recover from the surgery before SE induction. Lithium chloride (3 mM/kg, i.p.) was injected 12–24 h prior to the induction of SE. On the day of the EEG recording, the rat was placed in the recording cage and connected to a Grass-Telefactor EEG machine via a flexible cable suspended from the top of the cage, with an interposed commutator to allow rats to turn freely without twisting the cable. Following a baseline EEG recording of at least 1 h (Fig. 3), SE was induced by injecting pilocarpine (30 mg/kg, i.p.). EEG recordings were used to time the sacrifice depending upon the stage of SE desired. Control rats were not given pilocarpine.
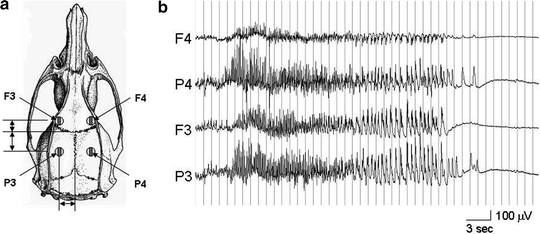

Fig. 3.
Example of an EEG recording from the status epilepticus animal model. (a) A scheme of four recording electrodes (F3, F4; P3, P4) was placed on the surface of skull. (b) The EEG recording shows a typical case of pilocarpine-induced status epilepticus (Stage-I).
3.2.2 EP Responses in Cat Cerebellar Cortex Evoked by a Selective Stimulation of Peripheral C-Type Nerve Fiber
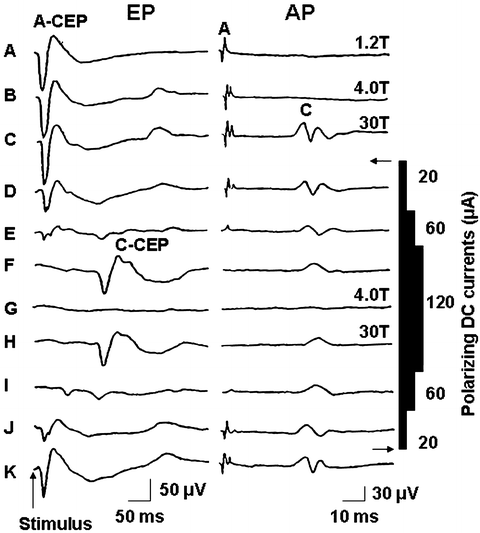
In this study, we ask whether selective C-fiber input to cerebellar cortex evokes a specific “slow-pain” response. The experimental procedure was the same as our previous description (12, 13). Briefly, adult cats were anesthetized using α-chloralose (60 mg/kg, i.v.). The trachea was canulated and both anterior and posterior lobes of cerebellar cortex were exposed after partial removal of the bony tentorium and occipital bone. The saphenous nerve was dissected free and its arterial blood supply left intact while the distal end was ligated and cut. Bipolar stimulation and recording electrodes were placed on the centripetal portion of the nerve from distal to proximal for monitoring of action potential volleys. A Ag-AgCl blocking electrode was placed between stimulation and recording electrodes to block A-fiber inputs. During the course of the experiment, the animal was paralyzed by injection with 4% Flaxedil (20 mg/kg, i.v.) and artificially ventilated. The rectal temperature and end-respiratory CO2 were continuously monitored and maintained within a normal physiological range. The saphenous nerve was electrically stimulated 20 times with single pulses (0.5 Hz and 0.2 ms) to obtain an averaged evoked potential. The stimulus strength was adjusted as a multiple of the threshold intensity (T) of A-type fibers as determined from nerve volleys. The intensities of the polarizing currents to selectively block A-fiber conduction were 30–120 μA.
Figure 4 shows an example of how selective activation of C-fibers induces an evoked potential in the cat cerebellar cortex. Stimulation of the saphenous nerve at 4 T strength, which excited the A-fibers alone, elicited A-fiber-evoked cerebellar field potentials (A-CEP) with a latency of 11.8 + 3.5 ms, which is similar to previous report (14). When the A- and C-fibers were activated simultaneously by increasing stimulus intensity to 30 T, the A- and C-fiber cerebellar evoked potentials (AC-CEP) could be recorded. The AC-CEP pattern was unchanged under these conditions (Fig. 4b, c). Figure 4d–j shows the effects of a polarizing current on the action potential of the saphenous nerve and the cerebellar evoked potential. By gradually increasing the intensity of the polarizing currents from 20 to 120 μA, the propagation of A-fibers was blocked but C-fiber input still remained by stimulation above C-fiber threshold (Fig. 4d–f). Under this condition, the A-CEP disappeared and a C-fiber-induced cerebellar evoked potential (C-CEP) was present and had a latency of 134.2 ± 18.4 ms (Fig. 4f). Reducing the stimulus intensity below C-fiber threshold prevented the elicitation of C-CEP (Fig. 4g). A gradual reduction in the polarizing blocking currents and stimulation at 30 T strength evoked the A-CEP but not C-CEP (Fig. 4i–k). These results suggest that stimulation of peripheral C-fibers caused a specific evoked potential response in the cerebellar cortex. However, without blocking A-fiber conduction, a similar stimulation of the peripheral nerve (activation of A- and C-fibers together) failed to induce this C-fiber evoked potential presumably because A-fiber input inhibited the C-fiber signals.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


