CHAPTER 19 Disorders of the Urinary System
ANATOMY
The urinary system of the horse, like that of most mammals, consists of paired kidneys and ureters, the bladder, and the urethra. With the exception of the abdominal portion of the urinary bladder, the entire urinary tract is located in the retroperitoneal space. In a newborn foal, each kidney weighs about 175 g. In an adult horse the left kidney weighs between 600 to 700 g, and the right kidney is usually 25 to 50 g heavier, although this is not a consistent finding, and one may observe the reverse relation.1,2 Thus the kidneys account for approximately 0.65% to 0.75% and 0.27% to 0.37% of the total body mass of the foal and adult horse, respectively.1,3 The right kidney is located immediately below the dorsal extent of the last two or three ribs and the first lumbar transverse process, is shaped like a horseshoe, and measures about 15 cm long, 15 cm wide, and 5 to 6 cm high (dorsal to ventral). Craniolaterally, the right kidney is embedded into the liver, and its more craniad position compared with the left kidney prevents it from being accessible on rectal palpation. Although not the classically bean-shaped organ found in human beings and small animals, the left kidney in the adult horse is more elongated than the right kidney, with the cranial pole at the level of the hilus of the right kidney; in equids, the left kidney is about 18 cm long, 10 to 12 cm wide, and 5 to 6 cm high. Because of its more caudal location, one routinely can palpate the caudoventral aspect of the left kidney during rectal examination. The blood supply to the kidneys comes from one or more renal arteries branching from the aorta. Accessory renal arteries (which generally enter caudally) may arise from the caudal mesenteric, testicular or ovarian, or deep circumflex iliac arteries.1,2
The ureters are 6 to 8 mm in diameter and travel about 70 cm to their insertions in the dorsal bladder neck or trigone, close to the urethra. The distal 5 to 7 cm of each ureter courses within the bladder wall. This intramural segment of the ureter functions as a one-way valve to prevent vesicoureteral reflux (VUR) with progressive bladder distention. The urinary bladder lies on the pelvic floor when empty but can increase in size and drop forward over the pelvic brim when filled with urine. The bladder can accommodate up to 3 to 4 L of urine before stimulation of micturition. In the foal the bladder is attached to the ventral abdominal wall by the urachus and remnants of the umbilical arteries. Consequently, when empty, the bladder is commonly a band-shaped structure in a neonatal foal. During the first few months of life, this ventral attachment loosens as the urachal remnant becomes the middle ligament and the umbilical arterial remnants become the round ligaments of the free border of the paired lateral ligaments of the bladder.1
The urethra is about 2 to 3 cm long in a mare and 75 to 90 cm long in a male. In the intact male the pelvic urethra, which is 10 to 12 cm long, widens in an elliptic pattern to a diameter of 5 cm across and 2 to 3 cm from dorsal to ventral. A rounded dorsal prominence, the colliculus seminalis, is located immediately caudal to the urethral orifice and is the site of the common openings of the ductus deferens and ducts of the seminal vesicles. The openings of the prostatic ducts are on two groups of small papillae lateral to the colliculus seminalis. Between 2 and 3 cm farther caudad, the ducts of the bulbourethral glands open in paired dorsal lines. The smaller openings of the ducts of the lateral urethral glands open at the same level on the lateral aspect of the urethra.1
A fibrous capsule that peels easily from the normal kidney covers the surface of each kidney. The equine kidney consists of an outer cortex slightly wider than the inner medulla. The cortex is dotted with dark spots—renal corpuscles or glomeruli within Bowman’s capsules. In horses, the corticomedullary junction is less distinct than in other species and is typically a deep red that contrasts well against the paler medulla and red-brown cortex. This region undulates along renal pyramids (cortex) and renal columns (medulla). The pyramids are subdivisions of the renal parenchyma, which are separated by arcuate arteries at the level of the corticomedullary junction. The equine kidney contains a total of 40 to 60 pyramids arranged in four parallel rows. The renal pelvis is the dilated proximal portion of the ureter. Microscopic examination reveals numerous small openings of the collecting ducts (ducts of Bellini). Additionally, the renal pelvis and proximal ureter are lined with compound tubular mucous glands and goblet cells that secrete thick, viscid mucus usually found in the renal pelvis and urine of normal horses.1,4
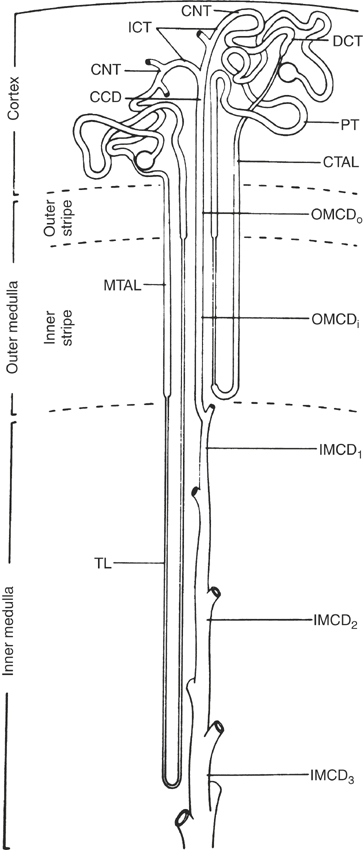
The functional unit of the kidney is the nephron. Each nephron is composed of a renal corpuscle (glomerulus within Bowman’s capsule), a proximal tubule (convoluted and straight components), an intermediate tubule (loop of Henle), a distal convoluted tubule, a connecting tubule, and cortical, outer medullary, and inner medullary collecting ducts (Figure 19-1). The two populations of nephrons are (1) the superficial (or cortical) nephrons possessing short loops of Henle and (2) the juxtamedullary nephrons with long loops of Henle. Gradations exist between these two general categories of nephrons, as well as species variation in the ratio of short-looped nephrons to long ones. For example, human beings have seven times more short- than long-looped nephrons, whereas essentially 100% of nephrons in dogs and cats have long loops.5 An early anatomic study found approximately 4 million glomeruli (nephrons) in the adult bovine kidney6; however, a recent study of kidney organogenesis using unbiased stereologic techniques to examine 45 equine left kidneys indicated that the left kidney of the horse may contain closer to 10 million glomeruli (for a total of 20 million in both kidneys).7 The latter study also confirmed that the total number of glomeruli does not increase after birth despite continued growth of the kidney until about 1 year of age. At present, little information is available on the ratio of short- to long-looped nephrons in horses. Histologically, equine nephrons are similar to those of other mammalian species; however, the diameter and epithelial height of the tubule and collecting duct segments are comparatively larger. In addition, the equine macula densa (segment of the ascending loop of Henle that lies in close association with the juxtaglomerular apparatus of the afferent arteriole) appears more prominent than that of other mammals.8 Whether or not these subtle histologic differences are accompanied by functional differences has not been investigated.
Relative to its size, the mammalian kidney has a richer innervation than almost any other organ.9 Although the neuroanatomy of the equine kidney has not been well studied, autonomic nerves course from the aorticorenal and renal ganglia along the major renal vessels into the kidneys.1 These nerves are predominantly sympathetic, because the kidneys appear to be poorly supplied by cholinergic nerves. Although the best-recognized effect of renal nerves is control of renal vascular resistance (for regulation of renal blood flow [RBF] over a wide range of perfusion pressures), the nerves also act directly on renal tubules and juxtaglomerular cells. For example, low-frequency stimulation of renal nerves (below the threshold for vasoconstriction) increases proximal tubular sodium reabsorption and renin release by activation of α1-adrenoceptors.10 In addition to α- and β-adrenoceptors, renal vasculature is rich in dopaminergic adrenoceptors, and activation of the latter, specifically dopamine type 1 receptors, leads to increased perfusion of the outer renal medulla. Presence of these receptors is the basis for use of dopamine, and more recently the DA-1 receptor agonist fenoldopam, in an attempt to improve RBF in acute renal failure (ARF) or to decrease the risk of radiocontrast nephropathy.11–13 The administration of drugs also can activate renal adrenoceptors unintentionally. A common clinical example is the diuresis induced by administration of the α2-agonists xylazine and detomidine. Although the diuresis has been attributed to a transient hyperglycemia and glucosuria, the latter is often absent.14,15 An alternative explanation may be drug binding to α2-adrenoceptors located on collecting duct epithelium. Activation of these receptors can lead to antagonism of the effects of antidiuretic hormone on cortical collecting ducts, which results in diuresis.16 More recently, renal afferent nerves have been identified, and these nerves appear to play a role in the pathogenesis of hypertension in species affected by this disorder.9
Autonomic innervation of the ureters, bladder, and urethra is important to ureteral peristalsis and micturition. The equine ureteral smooth muscle contains α1– and β2-adrenoceptors, which induce contraction and relaxation, respectively, when activated by norepinephrine.17 Recent studies of the innervation of the equine ureter demonstrated greater densities of adrenergic neurons in the proximal (renal pelvis) and intravesicular (bladder wall) portions of the ureter.18 Increased densities in these regions are consistent with the suspected pacemaker activity of the renal pelvis, which initiates ureteral peristalsis and the sphincterlike function of the distal segment of the ureter. The sympathetic nerve supply to the urinary bladder is provided via the hypogastric nerve, with preganglionic fibers arriving from spinal segments L1 to L4 to synapse in the caudal mesenteric ganglion. Postganglionic fibers supply the bladder (β2-adrenergic receptors) and proximal urethra (primarily α1– and some α2-adrenergic receptors).19,20 In addition to adrenergic innervation, cholinergic and peptidergic nerve fibers also innervate the equine bladder.21 Parasympathetic innervation originates in the sacral segments of the spinal cord, with neurons joining to form the pelvic nerve.19,20 Many complex interneuronal connections exist between sympathetic and parasympathetic nerves in the wall of the bladder, along with small adrenergic cells that facilitate interaction between sympathetic and parasympathetic pathways.22 As a result, complete denervation of the bladder is virtually impossible. Somatic innervation of the lower urinary tract is primarily to the striated muscle of the external urethral sphincter via a branch of the pudendal nerve, which originates from the sacral cord segments (S1 to S2).1
DEVELOPMENT
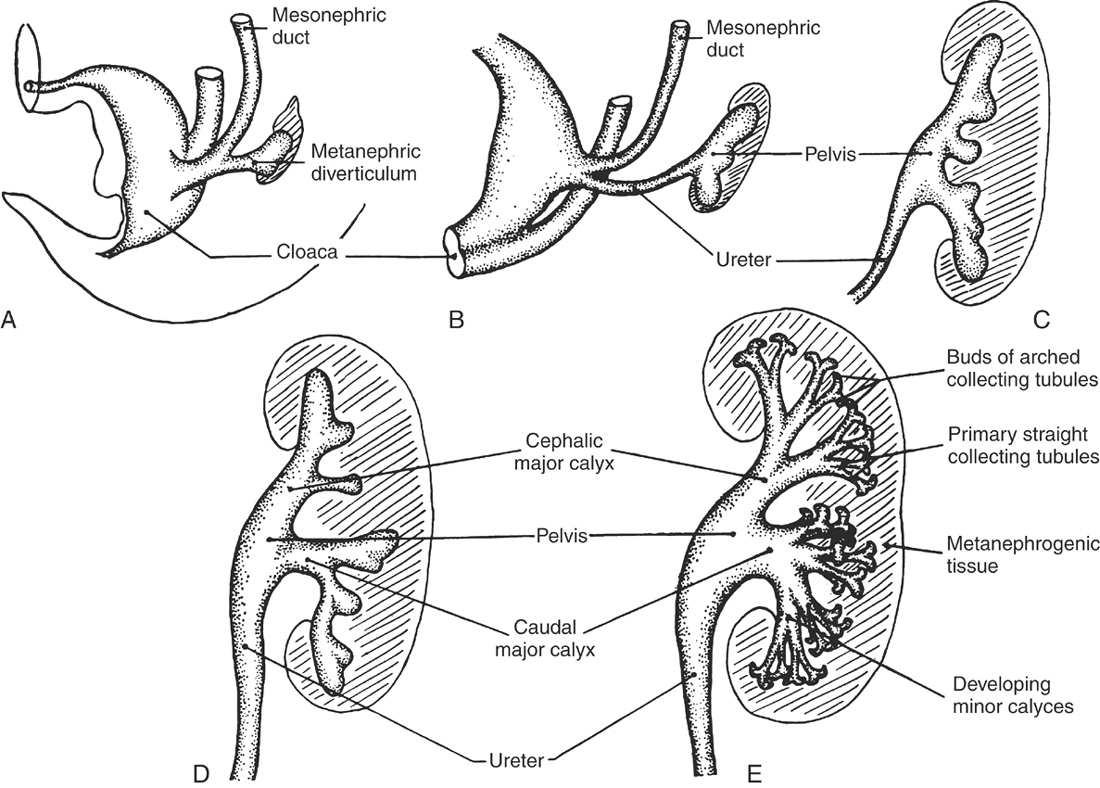
The embryonic upper urinary tract arises from bilateral primordial mesonephric ducts and intermediate mesoderm. The metanephric diverticulum originates from the caudal end of each mesonephric duct and develops craniad to become the ureter and renal pelvis. The advancing metanephric diverticuli collect about their ends intermediate mesoderm (metanephrogenic tissue), which becomes the collecting system and parenchyma of the mature kidney (Figure 19-2). The vascular supply is derived from a branch of the aorta (renal artery) that invades the metanephrogenic tissue. The urinary bladder develops as a dilated proximal portion of the allantois. The bladder is separated from the hindgut by the craniocaudad growth of the urorectal fold, which divides the rectum from the urogenital sinus. The latter structure gives rise to the urethra (Figure 19-3). The mesonephric and metanephric ducts initially open into the urogenital sinus, but as development continues, the distal segments of the mesonephric ducts are absorbed into the bladder wall and the openings of the metanephric ducts are pulled craniad to their final site in the dorsal bladder neck.23
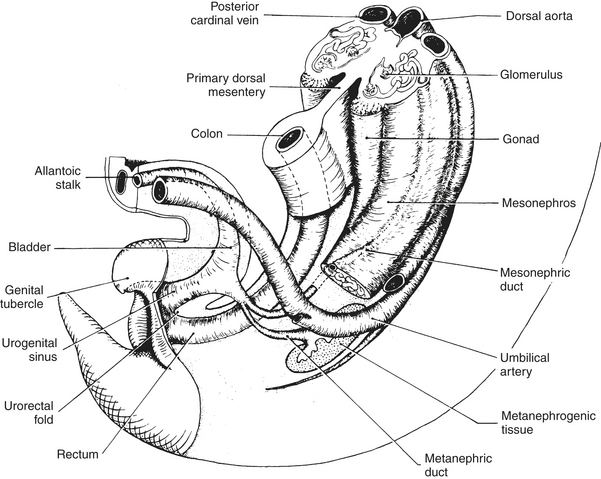
FIGURE 19-3 Developing urogenital tract of the young mammalian embryo.
(From Carlson BM: Patten’s foundations of embryology, ed 6, New York, 2003, McGraw-Hill.)
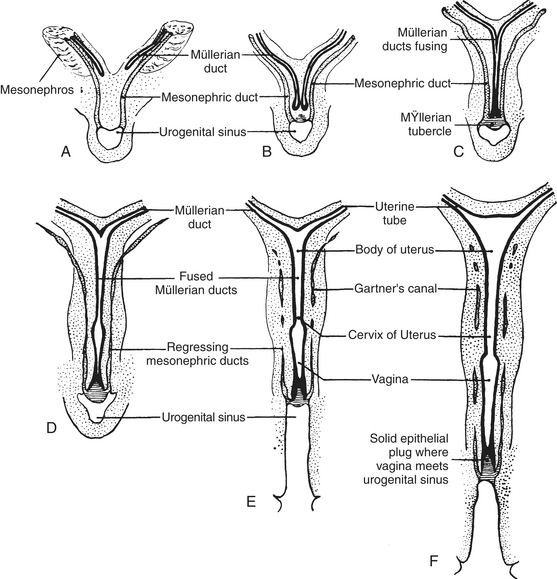
The fate of the mesonephric tubules (mesonephros) and mesonephric ducts varies with gender. Paired paramesonephric ducts (müllerian ducts) arise parallel to the mesonephric ducts in both sexes. In the female, the ducts fuse distally to become the vagina and uterine body, whereas proximally they remain separate to give rise to uterine horns and oviducts. The mesonephric ducts regress into vestigial remnants termed the epoöphoron proximally (near the ovaries) and Gartner’s canals distally (near the vagina and uterus; Figure 19-4). In the male, sexual differentiation of the gonads and production of androgenic steroid hormones lead to regression of the müllerian ducts. The duct system of the male reproductive tract is appropriated from the mesonephros and mesonephric ducts (also termed wolffian ducts). Androgenic steroid hormones also stimulate these structures to develop into the seminiferous tubules, epididymis, and ductus deferens. The distal portion of the mesonephric duct becomes the ejaculatory duct, the terminal portion of the ductus deferens.23
DEVELOPMENTAL MALFORMATIONS OF THE URINARY TRACT
Anomalies of the urinary tract are uncommon in horses. A survey by Höflinger24 revealed a similar frequency of unilateral renal agenesis (0.07%) in horses and human beings (0.10%).5 In contrast, horseshoe kidneys (attached at the cranial or caudal poles) are the most common anomaly in human beings (0.25%) but rarely have been described in horses.5,25
Renal Agenesis, Hypoplasia, and Dysplasia
Renal agenesis, which may be unilateral or bilateral, results from failure of the metanephric duct to fuse with the metanephrogenic mesodermal tissue. Although unilateral anomalies have been described more frequently, this simply may reflect the incompatibility of bilateral agenesis with postnatal life.24,26–28 Brown et al.28 described a foal with bilateral renal agenesis in which severe azotemia was detected shortly after birth. Bilateral ureteral dysgenesis and cryptorchidism, agenesis of the right adrenal gland, and atresia ani accompanied the renal agenesis in this foal. Unilateral defects may be incidental findings in otherwise healthy horses29 or may be detectable during examination of the reproductive tract, because many horses have associated anomalies of that system. Occasionally, unilateral agenesis may result in clinical renal disease if a problem arises in the contralateral kidney. Johnson et al.27 described a 4-year-old Quarter Horse with unilateral renal agenesis and a ureterolith causing contralateral hydronephrosis. The gelding was presented for weight loss, pollakiuria, and stranguria. In addition to the renal anomaly, unilateral agenesis of the ipsilateral testicle also was found on necropsy. Renal agenesis may be a familial disorder in several species.25,30 Although no information is available to suggest a hereditary basis in horses, one probably should discourage repeat matings after detecting such an anomaly.
One diagnoses renal hypoplasia when one kidney is at least 50% smaller than normal or when the total renal mass is decreased by more than one third.25 Renal hypoplasia is a quantitative defect caused by a reduced mass of metanephrogenic tissue or by incomplete induction of nephron formation by the metanephric duct. The condition may be confused with renal dysplasia. Unilateral renal hypoplasia usually is associated with contralateral hypertrophy and normal renal function, whereas bilateral hypoplasia generally leads to chronic renal failure (CRF).25,30 Andrews et al.31 described bilateral renal hypoplasia in a foal presented after death and in three young horses with CRF that had poor growth from birth. Anomalies in these four horses were limited to the upper urinary tract.
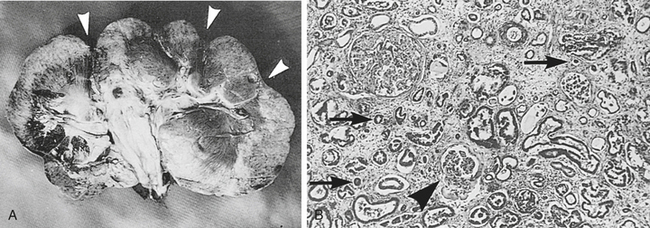
Renal dysplasia is disorganized development of renal tissue caused by anomalous differentiation, intrauterine ureteral obstruction, fetal viral infection, or teratogens.25,32 Bilateral dysplasia usually leads to renal failure. In general, dysplastic kidneys are normal in size unless concurrent hypoplasia exists or the animal lives for months to years before developing renal failure. Roberts and Kelly33 reported a case of bilateral renal dysplasia in a 19-month-old pony gelding. The pony was presented for weight loss over a 3-month period, and clinicopathologic assessment revealed CRF. A small, firm, and nodular left kidney was palpable per rectum. At necropsy, the kidneys weighed 280 g each (33% smaller than normal for body weight) and were nodular. Renal dysplasia was suspected because glomeruli in the collapsed areas of the kidneys were small, tubules were immature, and inflammatory cells were scant. Six similar cases of bilateral renal dysplasia resulted in CRF in horses from 2 months to 7 years of age.34–38 Small kidneys with increased echogenicity and an indistinct corticomedullary junction were typical ultrasonographic findings,36–38 and these findings were corroborated by computed tomography in one Miniature Horse foal.38 At necropsy, kidneys were typically small and irregular, the cortex and medulla were not well-delineated, and immature glomeruli and primitive tubules were found on histologic examination (Figure 19-5). Renal dysplasia also may cause renal failure in neonates. For example, Zicker et al.39 reported a case of renal dysplasia in a 2-day-old Quarter Horse foal presented for diarrhea and depression. Clinicopathologic assessment revealed azotemia, hyponatremia, hypochloremia, and urinary sodium wastage. At necropsy, the kidneys were normal in size (380 g), but histologic examination revealed immature glomeruli, hypoplastic tubules and vasa recta, and extensive myxomatous connective tissue occupying 90% of the total medullary volume. Finally, renal dysplasia also may be a unilateral problem that does not result in renal failure. Jones et al.40 found ureteropelvic polyps to be the cause of unilateral hydronephrosis and renal dysplasia in a Trakehner colt. Poor growth and hematuria for several weeks were the presenting complaints. Renal function remained normal for 8 months after nephrectomy until the colt developed a severe bout of colic, prompting euthanasia. Ureteral obstruction by the polyps was the suggested cause of renal dysplasia, because urinary tract obstruction has been found in a large percentage of cases of human renal dysplasia.32
Renal Cysts
One or more renal cysts occasionally are discovered as incidental findings on necropsy examination. The cysts may arise from any portion of the nephron but more often occur in the cortex than in the medulla. The pathogenesis is not known, but a defect in the basement membrane that allows tubular dilation is suspected. Renal cysts vary in size from microscopic to as large as the organ itself and routinely have a clear to slightly opaque wall and contain a thin, clear fluid. Congenital cysts are differentiated easily from acquired cysts (after obstruction) by the extensive scarring that accompanies the latter. Renal cysts also may develop as a consequence of drug therapy (i.e., long-acting corticosteroids) or exposure to certain chemicals.25,30
Polycystic Kidney Disease, Glomerulocystic Disease, and Other Hereditary Nephropathies
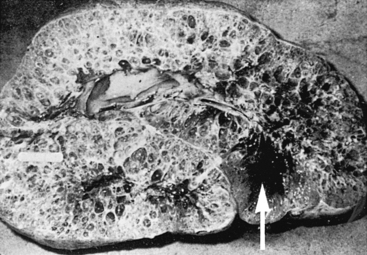
Polycystic kidney disease (PKD) is a disorder in which numerous, variably sized cysts are found throughout the cortex and medulla. With glomerulocystic disease, cysts are microscopic and limited to Bowman’s spaces. Cysts of the bile duct and pancreas also may occur with PKD, and both conditions have been described in stillbirths in many species, including foals.25 The two major types of human PKD are (1) a rare congenital or infantile form inherited as an autosomal recessive trait (which may be found in stillbirths) and (2) a more common adult form inherited as an autosomal dominant trait that leads to renal insufficiency in later life in association with dramatically enlarged, cystic kidneys.41,42 The latter form of PKD develops because of mutations in genes encoding for polycystins, integral membrane proteins responsible for cell-to-cell interaction.43 Autosomal dominant PKD also has been documented in Persian cats and related breeds and in bull terriers.44–46 The genetic defect in Persian cats is thought to be similar to the most common defect in human beings (PKD1 gene) and leads to end-stage renal disease by 3 to 10 years of age.45 As in human beings, the disorder is detectable by renal ultrasonographic screening of juvenile cats and preventable by avoiding subsequent mating of affected animals. Nevertheless, because of heavy inbreeding the prevalence of PKD in Persian cats and related breeds is between 40% and 50%.44,45
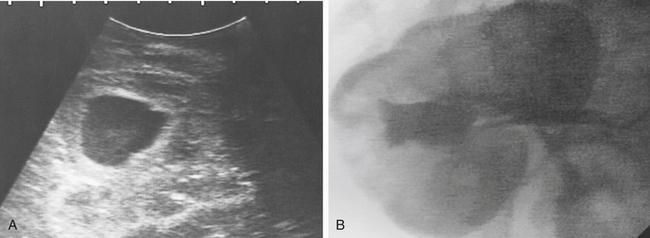
Ramsey et al.47 described polycystic kidneys in a 9-year-old Thoroughbred mare that exhibited anorexia and weight loss. Clinicopathologic assessment revealed CRF, and euthanasia was performed. At necropsy, the kidneys were grossly enlarged and each weighed 12 kg (Figure 19-6). A similar case of bilateral PKD was described in a 15-year-old pony with a 4-week history of hematuria and moderate weight loss. Evaluation revealed azotemia and presence of large masses in the area of both kidneys on rectal examination, and dramatically enlarged polycystic kidneys weighing 11.4 and 9.1 kg, respectively, were found at necropsy.48 Bertone et al.49 reported a third case of adult PKD in a 10-year-old Paint gelding with weight loss. The horse was mildly azotemic, and several 2- to 15-cm diameter cysts were imaged in both kidneys during ultrasonographic examination. In human beings, polycystic kidneys are believed to result in renal failure as cysts expand (sometimes under pressure) and compress adjacent normal renal tissue. Altered compliance of tubular basement membranes and proliferation of renal tubular epithelium result in outflow obstruction and proximal ballooning, leading to renal cyst formation.42 In some human cases, pressure within cysts may be 5 to 10 times higher than surrounding interstitial tissue pressures. Bertone et al.49 found no increase in pressure in several cysts catheterized percutaneously in a gelding with PKD, but differences in sodium concentrations suggested that the sampled cysts had arisen from different segments of the renal tubule. Euthanasia was performed after a prolonged hospital course (235 days), and the kidneys were not grossly enlarged except where distorted by large cysts. Although not well documented, PKD has been described anecdotally in two additional Paint horses, suggesting that an inherited form of PKD may occur in that breed. A recent report also documented PKD in an 11-year-old Andalusian gelding.50
In addition to PKD, a variety of other hereditary nephropathies have been described in human beings.48 Similar disorders are starting to be recognized in domestic animals, including hereditary nephritis in bull terriers, Samoyeds, and English cocker spaniels. Analogous to Alport’s syndrome in human beings, a defective molecular structure of type IV collagen, an important component of the glomerular basement membrane (GBM), appears to be the cause of hereditary nephritis in these dog breeds.46 Similarly, a syndrome of renal tubular dysplasia with autosomal recessive pattern of inheritance recently has been described in a population of highly inbred Japanese black cattle,51–53 as has a syndrome of suspected hereditary renal oxalosis in Beefmaster calves.54 Similar hereditary nephropathies are likely to occur in horses; however, to date the only one documented is a syndrome of nephrogenic diabetes insipidus in Thoroughbreds.55
Vascular Anomalies
Anomalies of the vascular supply to the equine urinary tract are rare but may result in hematuria, hemoglobinuria, partial ureteral obstruction, or hydronephrosis.30,56 Latimer, Magnus, and Duncan57 described a distal aortic aneurysm and associated extrarenal arterioureteral fistula in a 5-month-old colt presented for intermittent hematuria, colic, and lameness. Partial ureteral obstruction and hydronephrosis were observed on the affected side. Intrarenal vascular anomalies, termed renal arteriovenous malformations, are similarly rare (reported frequency of 0.04% in human beings).58 Interestingly, these vascular malformations may be silent until later in life, when varying degrees of hematuria and flank pain may ensue. The anomalous vessels are often tortuous and may be enlarged focally and devoid of elastic tissue. Hematuria and hemoglobinuria are thought to arise from areas where the anomalous vessels lie close to the collecting system.58,59 With vascular anomalies, one should attempt to determine the extent of the defect (unilateral or bilateral) via ultrasonographic examination, contrast radiographic studies, or cystoscopy (visualization that hematuria is coming from only one ureteral orifice). When a unilateral defect is documented in the absence of azotemia, unilateral nephrectomy or selective renal embolization has been recommended to prevent possible fatal exsanguination through the urinary tract56,57; however, one may consider conservative treatment if the urinary tract bleeding is minor and has not resulted in anemia.
A large vascular anomaly resulting in transient hemoglobinuria has been reported in a Quarter Horse colt.60 Over several weeks the large anomalous vascular structure (Figure 19-7) spontaneously filled with a thrombus so that specific treatment (a nephrectomy) was not pursued. Severe adult-onset, idiopathic renal hemorrhage also has been described in horses.61 Whether this latter syndrome may have been a consequence of congenital renal vascular malformations has not been determined (see Hematuria section later in this chapter). Occasionally gross hematuria with passage of blood clots can accompany omphalitis or bladder rupture.62 One usually can detect these problems during ultrasonographic examination of the umbilical structures and sometimes can image tissue echogenicity within the bladder that is attributable to a blood clot.
Pendulant Kidney
A pendulant kidney is a rare anomaly in the horse.63 Rectal examination reveals an extremely mobile kidney attached to the dorsal body wall by a thin band of tissue. Although a pendulous kidney could result from extreme weight loss, hydronephrosis, or perirenal trauma, the condition usually is thought to be congenital. The abnormality is an incidental finding unless displacement or rotation leads to partial or complete ureteral obstruction. As an example, the author has palpated the entire right kidney of one mare immediately craniad of the pelvic canal, and ultrasonographic imaging revealed normal size and structure of the anomalously located kidney.
Ectopic Ureter
Although ureteral ectopia occurs rarely in the horse,64 the condition is the most commonly reported developmental anomaly of the equine urinary tract.64–82 Ectopic ureters may develop when (1) the ureteric bud (metanephric duct) fails to be incorporated into the urogenital sinus or fails to migrate craniad to the bladder neck or (2) the mesonephric duct fails to regress. In the former case, the ectopic ureter opens near the urethral papilla in females or into the pelvic urethra near the colliculus seminalis in males, whereas in the latter, the ureter may open anywhere along the vagina, cervix, or uterus (but only in females because this portion of the mesonephric duct becomes the wolffian duct system in males). In 118 reported cases of ectopic ureter in horses, 105 (89%) were females65-68,72-77,79-82; however, this sex distribution may reflect easier recognition in females of the presenting complaint of urinary incontinence rather than a true sex predilection. Incontinence is recognized more often in females because urine entering the pelvic urethra in males may pass retrograde into the bladder. Although a genetic predisposition for ectopic ureter exists for several dog breeds,83 no breed predilection has been established in horses. However, Quarter Horses may be at greater risk because the condition has been reported in five Quarter Horses, three Standardbreds, two Thoroughbreds, two Appaloosas, an Arabian, a Clydesdale, a Shire, a Fresian, a Foxtrotter, and a Warmblood. The author also has seen the condition in two Quarter Horse fillies (one unilateral and one bilateral), yielding a total of 20 cases.
In horses with ureteral ectopia, urinary incontinence is generally apparent from birth, and affected animals are presented for extensive scalding of the hindlimbs. With unilateral ectopia, horses also void normally, because the other ureter enters the bladder in the appropriate location. Renal function is usually normal, but the affected ureter may be greatly dilated. Urine pooling in the vagina and uterus was a complicating factor in one case.73 To determine the site of the ectopic ureteral orifice (or orifices), one initially visually examines the vestibule and vagina (using a blade speculum) to look for intermittent urine flow from the area of the urethral papilla. Ectopic ureteral openings usually are not apparent unless urine flow is visible. Endoscopy may be helpful in females (while inflating the vestibule and vagina with air and using a hand to form a seal at the vulva) and is required in males to visualize the ectopic ureteral opening. Intravesical placement of methylene blue dye was performed in one filly to provide evidence for ureteral ectopia. Continued dribbling of clear urine (from the ectopic ureter) followed by passage of blue, discolored urine indicated that only one ureter emptied into the bladder but provided no information on the location of the opening of the ectopic ureter.66 Intravenous administration of dyes—including sodium fluorescein (10 mg/kg IV; yellow-green), indigotindisulfonate (indigo carmine, 0.25 mg/kg IV; blue-purple), azosulfamide (2.0 mg/kg IV; red), or phenolsulfonphthalein (1.0 mg/kg IV; red)—to discolor the urine may help locate ectopic ureteral openings.84 Contrast radiography (excretory urography or retrograde contrast studies via catheterization of the bladder and ureters) has been used to detail renal architecture and the course of the ureters in some affected animals; however, results of intravenous urograms are frequently inconclusive in foals weighing more than 50 kg (contrast agent is poorly imaged). In a recent report, ultrasound-guided pyelography, in which contrast agent was injected directly into the renal pelvis using a spinal needle, proved to be a more effective technique than imaging after intravenous administration of contrast agent to detail the course of an ectopic ureter, and one should consider this technique in future cases.82
Treatment has included ureterocystostomy (surgical reimplantation of the ectopic ureter or ureters into the bladder) or unilateral nephrectomy. Before surgery, one must determine whether the condition is unilateral or bilateral, which side is affected if unilateral, and whether urinary tract infection (UTI) is present. Further, one should attempt to rule out other anomalies, especially of the reproductive tract. If the problem is bilateral (8 of 20 cases), then one should establish the presence of a normal micturition response by measuring the intravesicular pressure response to progressive distention until the fluid infused is voided spontaneously. This procedure provides an estimate of bladder volume and ensures competency of the urethral sphincter before reimplantation. Among 14 cases in which surgical correction was pursued, ureterocystostomy was successful in establishing a functional urinary system in nine published cases∗ and one foal seen by the author, but four died of postoperative complications.68,75,82 In contrast, all four cases treated by unilateral nephrectomy had a favorable outcome.72,73,79 Because affected ureters often are dilated and tortuous, surgical reimplantation can be difficult and may not result in a functional ureteral orifice. Consequently, when the problem is unilateral, nephrectomy of the affected side may be the preferred treatment option.85,86
Ureteral Defects or Tears (Ureterorrhexis)
Retroperitoneal accumulation of urine and uroperitoneum has been described in seven foals with unilateral or bilateral ureteral defects87–93 and has been observed in three additional foals by the author. These included seven male and three female foals of several breeds (five Standardbreds, two Thoroughbreds, one Belgian, one Oldenburg, and one Appaloosa). Clinical signs (decreased nursing, depression, abdominal distention, diarrhea, and muscle twitching or other signs of neuromuscular irritability) and clinicopathologic abnormalities (hyponatremia, hyperkalemia, hypochloremia, and azotemia) are similar to those in horses with bladder rupture but may have a slightly later onset (4 to 16 days of age). Mild protrusion of the vagina may occur in fillies in which the peritoneum has remained intact.94 In affected foals, ultrasonographic examination may reveal dilation of the renal pelvis and affected ureter, as well as fluid accumulation around the kidneys or farther caudad within the retroperitoneal space. As with ectopic ureters, excretory urography generally has been an unrewarding diagnostic procedure, but contrast pyelography was used successfully to image leakage of contrast agent from a proximal ureteral defect in a recent report.93 Contrast radiography has not been pursued routinely because exploratory celiotomy generally was performed shortly after a diagnosis of uroperitoneum. Catheterization of the ureters via a cystotomy and retrograde injection of methylene blue allowed localization of the defect (or defects), and surgical correction was performed successfully in four cases by suturing the defect around an indwelling catheter.89,90,93 Although ascending UTI should be an expected complication with a stent, repair of a defect in one foal without use of an indwelling catheter resulted in further urine leakage from the ureter, prompting a nephrectomy 4 days after the initial surgery.91 Of the remaining five foals, one died after three unsuccessful attempts at surgical repair,88 and euthanasia was performed in four cases without attempting repair.87,92
At surgery or necropsy a single defect was found in six foals, whereas bilateral defects were found in four foals and multiple defects were apparent in one ureter. In most cases the defects have been located in the proximal third of the ureter near the kidney. Interestingly, distended, tortuous ureters, occasionally accompanied by hydronephrosis, also were described in three affected foals,88,91,93 and distal obstruction of the ureters at the bladder was suspected in two of these cases, prompting ureteroneocystostomy. Although several reports suggest that these ureteral defects may be anomalies of development, the actual cause of these ureteral defects is not known. Traumatic disruption was suggested in the initial report in which histologic examination of the margins of the defect revealed hemorrhage and proliferation of immature connective tissue.87 A traumatic cause was further supported by a subsequent report in which histologic examination of the defects revealed absence of transitional epithelium and inflammation in a foal that had been attacked by dogs.92 Inflammation and granulation tissue also were visible in the apparently obstructed distal ureter in one of the foals with ureteral distention, again suggesting an acquired lesion. Blunt abdominal trauma, often sustained during automobile accidents, can cause retroperitoneal accumulation of urine and uroperitoneum in human beings.95 Disruption of the ureter is usually near the kidney, and this complication of trauma may not be recognized for several days after injury. In one foal evaluated by the author, multiple rib fractures found at necropsy suggested that these ureteral tears actually could be a complication of foaling trauma.
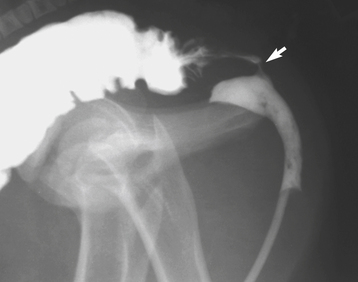
Rectourethral and Rectovaginal Fistulae
If the urorectal fold fails to separate completely the primitive hindgut from the urogenital sinus, then a rectourethral fistula may be found in a colt or a rectovaginal fistula or a persistent cloaca may be found in a filly.96 These anomalies are rare in horses and when present usually are associated with atresia ani and other anomalies, including agenesis of the coccygeal vertebrae and tail, scoliosis, adherence of the tail to the anal sphincter area, angular limb deformities, and microphthalmia.70,71,97–102 Affected foals usually are presented for atresia ani, although one also may observe signs of colic and straining. Evidence for a fistula is passage of fecal material from the vulva or urethra. In fillies one may detect rectovaginal fistulae by digital palpation of the dorsal vestibule and vagina, but in colts a definitive diagnosis usually requires contrast radiographic procedures such as a barium enema or a retrograde urethrogram (Figure 19-8). Surgical correction of atresia ani and fistulae has been performed successfully in several foals, but multiple surgical procedures may be required. Because ascending UTI may be a complication, one should submit a sample of urine collected via bladder catheterization (preferably during surgery) for bacterial culture.96 In human beings the evidence suggests that these anomalies are hereditary, and in one report several foals born with atresia ani were sired by the same stallion.97 Consequently, affected horses should not be used for breeding after surgical correction of the anomalies.
A urethrorectal fistula resulting in passage of urine from the anus also has been described in a 3-year-old Thoroughbred gelding.103 The fistula in this gelding was thought to be acquired after trauma or straining because no other developmental problems were detected and the edges of the defect were irregular and inflamed when examined with a speculum inserted into the rectum.
Bladder Defects
Uroperitoneum may result from bladder rupture during parturition in foals (most commonly males)104 or as a consequence of urachal leakage after infection of the umbilical structures.105,106 In addition, Wellington described uroperitoneum in two foals that were full brothers.107 Urine entered the abdomen from a dorsal defect in both colts, and smooth margins to the defects combined with a lack of appreciable inflammation provided evidence in favor of anomalous development rather than trauma. Other authors have suggested that some cases of uroperitoneum likely are associated with anomalous bladder defects because of the size, location, or lack of apparent inflammation of the margins of the defects.70,108–111 For example, Bain108 described uroperitoneum in a foal in which the ventral portion of the bladder was absent between the lateral ligaments (umbilical artery remnants) from the umbilicus to the urethra.
Anomalous fusion of the bladder to the inner umbilical ring (absence of the urachus) has been described in one foal.112 The malformation precluded normal contraction and evacuation of the bladder, and a megavesica—a greatly enlarged bladder—developed. The clinical appearance was similar to that of uroperitoneum, and surgical separation of the bladder from the umbilical ring restored normal anatomic and functional integrity of the bladder. A similar case with a greatly distended bladder was reported in a foal evaluated for abdominal distention70 that was attributed to an adhesion of the bladder to the urachus or umbilical remnant. An enlarged, flaccid bladder also was described in a foal undergoing exploratory celiotomy for suspected urinary tract disruption.105 Adhesions to the abdominal wall were not reported, and the foal survived after the surgery during which 50% of the distended bladder was resected. In addition to bladder distention, persistent attachment of the bladder to the area of the umbilicus via a urachal remnant was reported to cause pollakiuria and dysuria in a 15-month-old Thoroughbred filly.113 The author also has seen postpartum bladder rupture in a mare in which a persistent urachal attachment was suspected to be a contributing factor.
Excessive bladder distention or megavesica has been described further in four stillborn foals114 and one neonatal foal.115 In the latter foal and in another report,116 chronic bladder distention appeared to lead to loss of smooth muscle in the dorsal bladder wall and replacement with collagen. The result was bladder rupture during parturition. Although these reports are similar to an early report by Rooney104 describing the dorsal bladder wall as the anatomic weak link and likely area for rupture, they differ in that chronic distention of the bladder in utero with smooth muscle loss is not recognized in more typical bladder ruptures in neonatal foals. Why bladder distention should occur in utero without obstruction of the lower tract (not found in these cases) is not clear. Although an excessively long umbilical cord (longer than 85 cm) may lead to urachal obstruction,114,117 urine produced in utero alternatively could drain into the amniotic cavity via the urethra. Thus this form of megavesica remains poorly characterized and poorly understood.
Some neonatal foals examined for abdominal pain are apparently unable to urinate normally. Ultrasound examination will reveal a normal gastrointestinal tract and a greatly enlarged but intact urinary bladder. These foals can be treated with indwelling urinary catheters for 2 to 3 days along with phenazopyridine (4 mg/kg PO every 8 to 12 hours) for 5 to 7 days.118 Phenazopyridine acts a local analgesic on the lower urinary tract and may relieve spasm or allow better relaxation of the bladder sphincters and promote normal micturition.
Patent Urachus
The urachus is the conduit through which fetal urine passes from the bladder into the allantoic cavity. Normally, the urachus closes at the time of parturition, but incomplete closure is the most common malformation of the equine urinary tract. Patent urachus occurs more commonly in foals than in other domestic species.30 Greater than average length or partial torsion of the umbilical cord has been suggested to cause tension on the attachment of the umbilical cord to the body wall. The result is dilation of the urachus and subsequent failure to close at birth.70,71,114,117,119 Patent urachus results in a persistently moist umbilicus after birth, from which urine may leak as drips or as a stream during micturition. One must distinguish this malformation from septic omphalitis, which also can result in urine leakage from the umbilicus within a few hours to days after birth. Ultrasound examination of the umbilical remnants is indicated to rule out or monitor for concurrent omphalophlebitis. Patent urachus has been referred to as a congenital problem and the latter as an acquired problem, but both may result in urine leakage from the urachus from birth. Neither is life threatening, but local sepsis often is accompanied by more severe illness, including septicemia or localized infection, particularly in joints.
The congenital patent urachus traditionally has been treated with frequent (two to four times daily) chemical cauterization of the urachus with swabs dipped in a concentrated phenol or 7% iodine solution or with silver nitrate applicators.69 Because the urachus may close spontaneously in a number of cases, and because these agents desiccate and irritate tissue (and may predispose to infection), the rationale for this approach has been questioned.119 In a study comparing the effects of disinfectant solutions on the bacterial flora of the umbilicus of normal foals, use of a 7% iodine solution was observed to cause rapid desiccation of the umbilical tissue and subsequent development of a patent urachus when the stump fell off a few days later.120 Chlorhexidine diacetate (0.5%) appears to be more effective than 1% to 2% iodine solutions in reducing umbilical bacterial counts. Chlorhexidine is less irritating to tissue, binds to the stratum corneum, and has prolonged antiseptic effects.120 The use of caustic or irritating solutions for routine umbilical dipping or treatment of patent urachus should probably be avoided because it may lead to localized necrosis and infection that may actually result in umbilical infections.
Consequently, in the absence of apparent infection, no local treatment may be indicated specifically, but affected foals frequently are given antibiotics prophylactically. For acquired patency (which may be associated with local infection, cachexia, or septicemia), broad spectrum antibiotic therapy is indicated, and resolution of the systemic disease may be accompanied by elimination of the umbilical infection and closure of the urachus. Most cases of patent urachus without septic omphalophlebitis will resolve with supportive care, routine umbilical disinfection, and antibiotic therapy. Empiric antibiotic therapy with antibiotics that are eliminated (and concentrated) in urine, such as potentiated sulfonamides, cephalosporins, aminoglycosides, or penicillins usually provides adequate antimicrobial protection with uncomplicated patent urachus in otherwise healthy foals. Chemical cauterization is contraindicated with local sepsis because it may increase the risk of urachal rupture and development of uroperitoneum.121 If one observes no decrease in urine leakage after 5 to 7 days of medical therapy, or if ultrasonography reveals abnormalities of multiple structures in the umbilicus,122,123 then surgical exploration and resection of the urachus and umbilical vessels may be indicated. In a retrospective study of 16 foals treated for sepsis of umbilical cord remnants, six of nine (67%) survived after surgical resection and antibiotic treatment, whereas only three of seven (43%) survived after antibiotic treatment alone.124 Although this series of 16 foals often is cited in support of surgical intervention, one should note that the series studied a small number of foals and that the cases were evaluated over 10 years (1975-1985), during which time many aspects of neonatal care improved. In a more recent retrospective report of 33 foals with umbilical remnant infections, no difference in survival was observed between foals treated with antibiotics combined with surgical resection or with antibiotic therapy alone.123 Further, emphasis was placed on the insensitivity of palpation of the umbilicus in detection of umbilical remnant infection (compared with ultrasonographic examination) and the poor outcome of cases in which the umbilical vein was involved. In addition to the possibility of omphalitis leading to urachal rupture and development of uroperitoneum, urachal leakage also may occur into the abdominal musculature and subcutaneous tissues and lead to swelling and cellulitis of the ventral abdominal wall.125 Both instances require surgical intervention. Finally, trauma or tearing of the urachus also can lead to umbilical evagination of the urinary bladder,126 which can result in partial or complete obstruction of urine flow, and surgical correction is indicated.
PRODUCTION AND ELIMINATION OF NITROGENOUS AND ORGANIC WASTES
The two most commonly recognized waste products excreted in urine are urea and creatinine (Cr), but many other nitrogenous or organic wastes are produced each day and subsequently are eliminated by the kidneys (Box 19-1).1
Urea Metabolism
A molecule of urea is produced in the liver from two ammonium ions that are liberated during catabolism of amino acids. For each urea molecule the carbon atom is derived from bicarbonate. One ammonium ion is cleaved from an amino acid via an α-ketoglutarate–dependent transamination coupled to oxidative deamination of glutamate. The second ammonium ion is derived from aspartate in the urea cycle.2 Urea synthesized in the liver is released into the blood, and clearance by the kidneys represents the major pathway (75% to 100%) of excretion. Extrarenal urea excretion includes losses in sweat and through the gastrointestinal tract. With normal intestinal function, enteric excretion is minimal because of enterohepatic recirculation (reabsorption of ammonia from the degradation of urea by bacterial ureases and subsequent reformation of urea in the liver).3
In human beings, inborn errors of metabolism leading to deficiency of a specific transaminase or of one of the five enzymes of the urea cycle can result in accumulation of ammonia and other intermediates of amino acid catabolism. These disorders typically are inherited as autosomal recessive traits, and the consequence is moderate to severe mental dysfunction because the accumulated intermediates can be toxic to the central nervous system (CNS; i.e., ammonia) or can act as false neurotransmitters (i.e., aromatic amines).1 Because urea production is limited in these disorders, blood urea nitrogen (BUN) concentration is often low.2 Although such defects in metabolism appear to be rare in domestic animals,4 development of encephalopathy in association with hyperammonemia has been recognized in horses.5,6 Furthermore, in one report of two related Morgan weanling fillies, persistent hyperammonemia was suspected to be caused by a defect in a mitochondrial ornithine transporter similar to an autosomal recessive syndrome of hyperornithinemia, hyperammonemia, and homocitrullinuria (HHH) in human beings.7
BUN concentration depends on age, diet, rate of urea production, and renal function. For example, a low BUN typically is found in neonatal foals after an anabolic demand for amino acids.8 Investigations of nitrogen use in ponies have demonstrated that urea production is proportional to dietary protein content. Similarly, urinary urea excretion increases in proportion with urea production.9,10 As a result, with increased levels of dietary protein or when urea is supplemented in the diet, BUN may increase twofold or greater.11–13
In human beings and small animals, BUN is routinely higher in samples collected postprandially because diets are typically high in protein.3 Postprandial elevation of BUN has not been described in horses or other herbivores. However, fasting leads to enhanced protein catabolism to meet energy demands and increased BUN in horses.14,15 In ponies, however, BUN decreases with fasting.16 This opposite response suggests differences in the metabolic responses of horses and ponies to anorexia, consistent with a greater capacity of ponies to mobilize and use fat during starvation. Other causes of protein catabolism, including fever, infection, trauma, myositis, burns, and corticosteroid therapy, also can produce an increase in BUN.3 Finally, a decrease in RBF or renal function produces an increase in BUN. The former may occur with dehydration or during periods of anesthesia or exercise; the latter is a reflection of renal disease.3 With short bouts of moderate to intensive exercise, BUN often does not change13,17; however, during prolonged exercise BUN can increase by 50% or more because of the combined effects of decreased RBF and protein catabolism.18,19
Most renal nitrogen excretion occurs in the form of urea in urine. One must recognize that urea excretion is completely passive and that the high concentrations achieved in urine are merely a consequence of medullary tonicity produced by the countercurrent-multiplier function of the loop of Henle. Thus although variations in dietary protein intake lead to parallel changes in urea excretion, the idea that low-protein diets decrease the workload on the kidney is a fallacy.3 Urinary urea nitrogen concentrations can vary from as low as 50 mg/dl in neonatal foals or horses with primary polydipsia to greater than 2500 mg/dl in normal horses on high-protein diets. Total daily urea excretion usually ranges between 100 and 300 g per day in horses with normal renal function.
Creatinine Metabolism
Cr is produced by the nonenzymatic, irreversible cyclization and dehydration of creatine. Three amino acids in the kidney, liver, and pancreas are indirectly responsible for its production; subsequently Cr is transported to other organs such as muscle and brain, where it is phosphorylated to store energy in the form of phosphocreatine.3,20 In human beings, 1.5% to 2% of the creatine pool is converted to Cr daily and results in fairly constant excretion of Cr within a given individual.3 With normal renal function, a direct relationship exists between daily Cr production, serum Cr concentration, and Cr excretion, all three being proportional to total muscle mass. The fact that Cr is 30% higher in male humans than in female humans and that urinary Cr excretion is correlated to body size across a wide range of animal species supports this relationship.3,21 Cr is excreted principally in urine, but sweat and the gastrointestinal tract are secondary routes of excretion.3 In contrast to urea, enterohepatic recycling of Cr does not occur, and the gastrointestinal tract may represent a major route of excretion when renal function is compromised. For example, in a group of azotemic human patients, between 15% and 65% of radiolabeled Cr was found to be excreted through the intestine.22 Cr excreted by this route is degraded rapidly by bacteria so that little is found in feces.
Like BUN, Cr can vary with age, activity level, and renal function. In contrast, dietary protein intake has little influence on Cr in horses.11 Newborn foals routinely have Cr values 30% to 50% higher than those measured in the mare, and values as high as 20 to 30 mg/dl have been measured in some premature or asphyxiated foals.8 These high values may result from limited diffusion of Cr across the placenta. For example, the Cr in equine amniotic fluid collected at term is proportionately much greater than urea nitrogen concentration (Cr, 10.1 mg/dl; urea nitrogen, 38.8 mg/dl).23 If the foal appears healthy and all other laboratory values are within reference ranges, then a Cr value in the range of 5 to 15 mg/dl should not cause alarm. In most healthy foals with normal renal function, Cr decreases to values below 3.0 mg/dl within the first 3 to 5 days of life.22 After the first few days of life, Cr is usually lower in foals than in adults12 because of the combined effect of rapid growth and the fact that skeletal muscle comprises a smaller percentage of body weight in foals than in adult horses. Other nonrenal factors that may influence Cr include fasting, rhabdomyolysis or muscle wasting caused by disease, and exercise. Although fasting can increase the measured value for Cr, a substantial portion of this increase actually is due to other compounds (possibly ketones) that increase during fasting and are measured as non-Cr chromagens in the commonly used Jaffe’s colorimetric assay for Cr determination (see Examination of the Urinary System).12,14,24
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree