CHAPTER 13 Disorders of the Skin
BASIC STRUCTURE AND FUNCTION OF THE SKIN
GROSS ANATOMY
Skin
Skin thickness varies between 1 to 5 mm depending on the body location. The skin thickness is greatest at the dorsum and the proximal extremities and gets thinner ventrally and distally down the extremities. Skin is thickest on the forehead, dorsal neck, dorsal thorax, and base of the tail. The skin is thinnest on the ears and in the axillary, inguinal, and perianal areas.2,3
The skin consists of two main layers: the epidermis and dermis. The epidermis consists of the basal, spinous, granular, clear, and horny layers. Each layer has different types of cells and differs in function. The basal layer consists of a single column of cells that are attached to the basement membrane. Typical basal keratinocytes have an oval nucleus, prominent nucleolus or nucleoli, and little heterochromatin. The basal keratinocytes contain melanosomes; various metabolic and synthesizing organelles such as mitochondria, lysosomes, rough endoplasmic reticula, and Golgi complexes; and cytoskeletal structures such as keratin intermediate filaments, microfilaments, and microtubules. Structures such as desmosomes and hemidesmosomes that help form the attachment and help to shape the basal layer are also present.4,5
The spinous layer of the epidermis comprises two to four layers. The cells become progressively differentiated as they move outward toward the skin surface. The spinous layer cells first appear polyhedral and then become more flattened as they develop and move outward. The spinous cells contain cellular organelles and keratin filaments. The cells of the uppermost one to two layers of the granular layer contain small, oval, membrane-bound organelles (300 nm) with alternating lamellae called lamellar granules (membrane-coating granules, keratinosomes, Odland bodies) formed in the Golgi region. They contain polar lipids such as phospholipids, glycosphingolipids, free sterols, and so-called probarrier lipids and hydrolytic enzymes that convert the probarrier lipids into stacks of neutral lipid-rich lamellae that assemble in the intercellular space, coat the surfaces of the cornified cells, and provide a barrier to the permeation of the skin. The spinous cells have abundant desmosomes that consist of adhesion plaques and submembranous plaques involved in cell cohesion.4,5
The granular layer consists of highly differentiated cells that are flattened polyhedrons with keratohyalin granules. In this layer of the skin, dephosphorylation and proteinolysis activate filaggrin, which cross-links and bundles with keratin filaments into large macrofilaments. Filaggrin is broken down in the stratum corneum to free amino acids important for the proper hydration of the epidermis. Lamellar granules present in the granular layer aggregate beneath the plasma membrane and fuse with it, opening a channel for the release of granule contents such as polysaccharides, glycoproteins, acid hydrolases, acid lipase, and probarrier lipids into the intercellular spaces. After release, polar lipids are remodeled into hydrophobic, neutral, lipid-rich lamellae that form an effective barrier. A dense layer of highly cross-linked proteins is deposited inside the inner leaflet of the membrane. Together with the membrane, this boundary is called the cornified cell envelope, a rigid structure that resists degradation and is rich is glutamyl-lysine isopeptide cross-links. At the uppermost aspect of this layer, substances such as keratins, filaggrin, and the cornified cell envelope are passed on to the cornified cell layer.4,5
The cornified layer contains the largest and most numerous cells of any zone in the epidermis. Cells are large, flattened, and polyhedral; overlap with the margins; and associate through interlocking ridges and modified desmosomes. The contents of the cell are limited primarily to disulfide-bonded keratin filaments, highly cross-linked bundles of macrofilaments, and filaggrin. The compact organization of the proteins becomes looser toward the outer epidermal surface. The breakdown of filaggrin to its constituent amino acids in the outer cell layers accounts for the greater water-holding capacity of the cells and the more diffuse organization of the filaments. Uricanic acid is degraded from filaggrin with histidase and absorbs ultraviolet light. Therefore uricanic acid is important in ultraviolet light protection. The pyrrolidone carboxylic acid derived from glutamine is hygroscopic and helps to keep the skin hydrated even in a dry environment. The preserved trilaminar plasma membrane outside the cornified cell envelope becomes discontinuous and desquamates toward the upper portion of the cornified layer so that the envelope serves as the real cell membrane.4,5
The dermis contains a variety of cells that are essential for the skin to function normally. The dermis contains cellular elements such as fibroblasts, macrophages, histiocytes, eosinophils, and mast cells in the fibrous matrix and nonfibrous matrix. The fibrous matrix consists of collagen and elastic fibers. The nonfibrous matrix consists of glycosaminoglycans and proteoglycans. The blood vessels, nerves, lymphatics, hair, and sebaceous and apocrine glands are found within the dermis. The sebaceous and apocrine glands in the horse are larger and more numerous compared with those in other large animal species.4,5
The area that separates the epidermis from the dermis is known as the basement membrane zone. This layer of the skin functions as a scaffold for organization (important for growth and differentiation of keratinocytes) and repair (speeds up reepithelization), acts to regulate selective permeability by only allowing passage of molecules of the correct charge and molecular size, provides a physical intercellular barrier (tumor containment), and links epithelia to their underlying matrices. The basement membrane consists of structures such as tonofilaments, hemidesmosomes, laminin, fibronectin, proteins such as collagen, laminin-like molecules such as entactin and nidogen, anionic glycosaminoglycans such as heparin sulfate, anchoring fibrils, oxytalan filaments, elaunin fibers, microthread-like filamentous threadwork, and large glycoproteins such as fibrillin.5
Hair
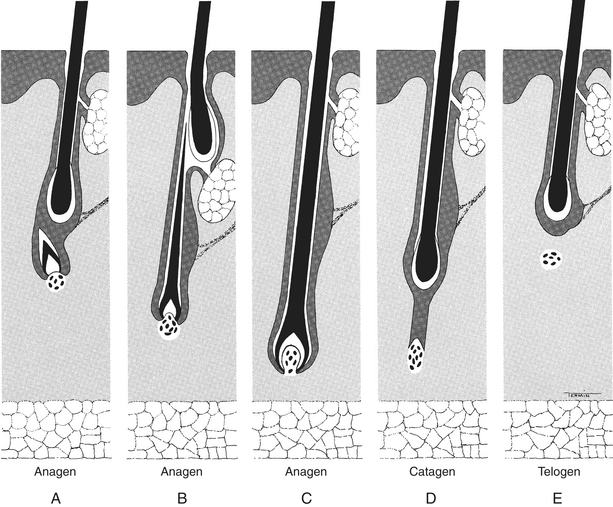
The hair tends to cycle in a specific manner. Initially, hairs are in the growing or anagen phase, progressing through an intermediate or catagen phase into a resting or telogen phase (Figure 13-1). The time period that hairs are in the catagen phase is short, and finding hairs in this stage of the cycle is difficult using a trichogram (plucking hairs and looking at them under the microscope). A variety of conditions including temperature, photoperiod, genetics, and hormones can affect the hair cycle.1,6
The hair follicles are located within the dermis and are associated with sebaceous and apocrine glands. Hairs are important in thermoregulation, protection of the skin from external factors, and as an ornament to attract other animals. The types of hairs found in equine skin are simple primary hairs. Horse hairs tend not to shed at once. Instead their hair sheds in patches or in a mosaic pattern.1
Ambient temperature affects the texture of the hairs. In warm temperatures the hair coat comprises thick, medullated hairs. Piloerection of hairs occurs to aid in cooling the body. In contrast, in cold temperatures the coat comprises longer, finer, and poorly medullated fibers. This type of hair coat provides more insulation from cold. The hairs of the fetlock, mane, and tail do not shed as do the other hairs on the horse.1
HISTORY
A patient history of the horse can provide the clinician with useful clues to help determine the underlying cause of a dermatologic problem. Figure 13-2 is an example of a form with different types of dermatologic questions. For example, most allergies produce the clinical sign of pruritus. The owner may notice the horse rubbing, scratching, licking, or chewing, or physical examination may reveal evidence of pruritus (i.e., broken hairs and excoriations). Pruritus does not confirm a diagnosis of allergic disease. Other conditions such as parasitic infestation or secondary bacterial or fungal skin infection may be pruritic. These differential diagnoses may be ruled out by performing the diagnostic tests discussed later in this chapter.
Questions concerning whether other horses in the stabling or pasture facility have dermatologic lesions may be important. Similarly, knowing if grooming aids (brushes, combs, etc.) are used on more than one horse is important. Pruritus that affects multiple animals may indicate the possibility of a parasitic or infectious process.
PHYSICAL EXAMINATION
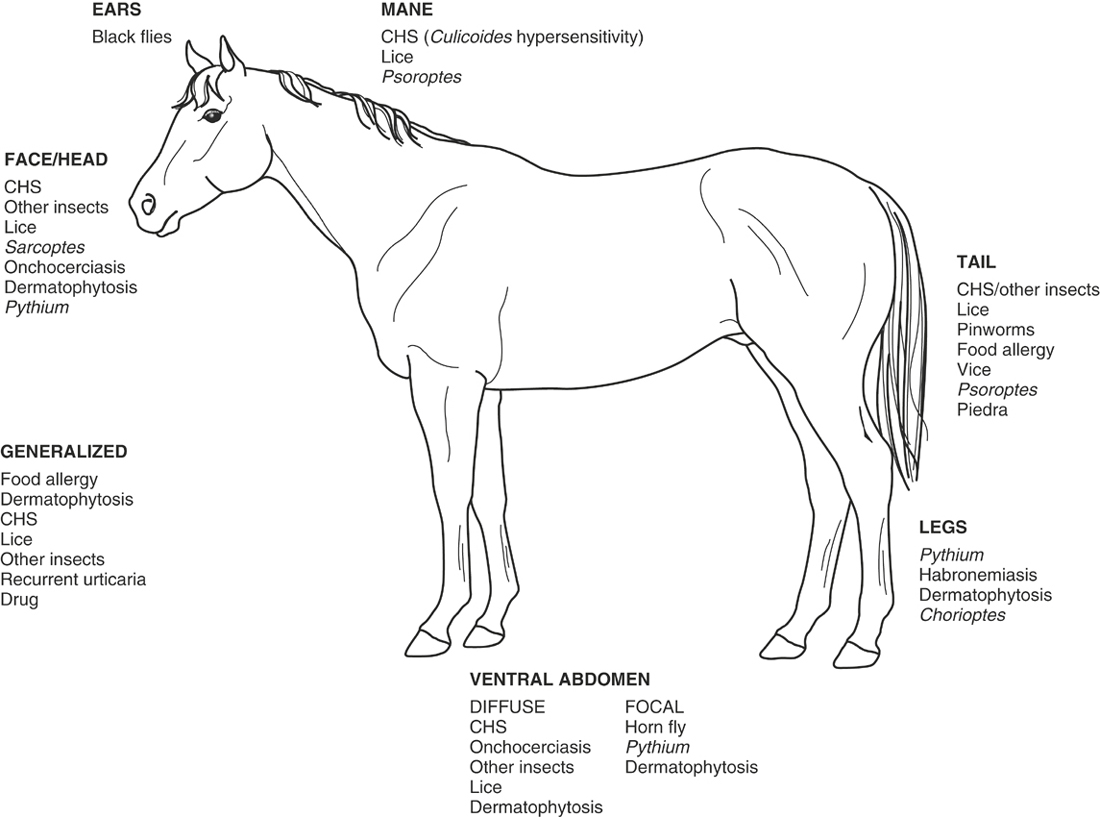
The location of lesions is important because certain dermatologic conditions tend to occur on certain areas of the body. This tendency is especially true with parasitic hypersensitivity reactions because certain parasites have a predilection for certain areas of the body.7 For example, pinworms tend to cause intense pruritus of the tailhead, and black flies tend to have a predilection for the head, ears, and ventral abdomen. More detailed information concerning insect feeding preferences is covered in the insect hypersensitivity section. The horse should be examined for evidence of external parasites such as ticks or flies.
The clinician should always perform a thorough physical examination, including determining the temperature, heart rate, and respiratory rate. If abnormalities in any of these parameters exist, then the horse may have a systemic disease or more than one medical problem. For the health of the animal, exploring and treating all possible medical problems is important.
COMMON CAUSES OF PRURITIC DERMATOSES
Pruritus is the sensation to rub, lick, scratch, or chew.8 Horses most commonly rub the skin and hair coat when they are pruritic (Figure 13-3). Clinical evidence that a horse is pruritic may include the presence of broken hairs, excoriations, hemorrhagic crust, alopecia, lichenification (thickening of the skin), and hyperpigmentation. Lichenification and hyperpigmentation occur most commonly after chronic inflammation and pruritus.
Parasitic Skin Diseases
LICE (PEDICULOSIS)
Lice infestations can occur year round, but they are more common in the winter in northern climates. Cooler skin and hair coat temperatures are more favorable for development of eggs within the female, oviposition, and egg development.9 Skin temperatures on the legs of horses, with the possible exception of Draft-breed horses, are too cold for louse development.
Lice infestation has no age, breed, or sex predilection in horses. Affected horses tend to be restless and have a poor appetite. They have a dry, dull hair coat with patchy areas of hair loss and excoriation. The coat may appear moist and have a sour or mousy odor. Skin fasciculation may be the only evidence of early infestations in foals. Some horses may be asymptomatic carriers that are identified only when foals or other susceptible animals become infested. Heavy infestations of sucking lice may cause anemia or severe debilitation.
The diagnosis of lice infestations is made by demonstration of adults or eggs or both on the hairs. Sometimes, lice cannot be visualized easily in direct light. In these circumstances the veterinarian may need to comb the hair coat with a fine-toothed comb to find the lice. Skin biopsies may reveal a nonspecific superficial perivascular eosinophilic dermatitis with or without intraepidermal microabscesses.1
When managing a lice infestation, the veterinarian should treat all animals on the premises. Ivermectin at 200 μg/kg orally every 2 weeks for three treatments is effective for treating sucking lice. Biting lice do not respond to ivermectin treatment, and thus one should treat affected horses with other medications, such as dips. Common types of dip may be used to treat sucking and biting lice infestations include lime sulfur, pyrethrins, methoxychlor, malathion, coumaphos, crotoxyphos, pyrethroids, lindane, permethrin, selenium sulfide, imidacloprid, phoxim, and fipronil.10–12 Lime sulfur and pyrethrin dips have fewer associated adverse reactions in horses than do other dip treatments. All products should be used according to label directions. None of these treatments affect lice eggs, and two to three repeat applications of the dip are recommended at 2-week intervals. For optimal efficacy, one should treat the whole body of the horse when applying the dips. Other precautions include cleaning and disinfecting all tack and grooming supplies (brushes and combs), decontaminating the stabling area using a commercial premise spray used to kill fleas because lice may survive for days to weeks in the environment, and, although reinfestation is difficult to prevent, treating animals returning from shows, breeding farms, or training facilities prophylactically.
MITES (MANGE)
A variety of mites may infest horses. Sarcoptes scabiei var. equi (scabies, head mange), Chorioptes equi (leg mange), Psoroptes equi (body mange), Pyemotes tritici (straw itch mite), and Trombicula and Eutrombicula species (chiggers) are species associated with equine pruritic skin disease.7 The pruritus accompanying a mite infestation results from a combination of mechanical irritation and hypersensitivity to the mite and by-products of the mite (i.e., feces).13 The primary skin lesion from a mite infestation is a maculopapular eruption. These skin lesions are often difficult to find with chronic infestations.
Sarcoptes scabiei
In the United States, S. scabiei infestations in horses are a reportable disease.7 This mite is highly contagious and infests horses and transiently infests human beings (self-limiting disease) in contact with parasitized horses. This parasite is capable of surviving off the host for up to 3 weeks, and transmission of scabies by fomites or in the environment is possible. Scabies mites burrow in the superficial epidermis and lay their eggs. In early infestations, mites are found in highest concentration around the head and neck. These mites seem to prefer the ears on horses. As disease progresses, mites usually spread over the entire body. A scabies infestation in a horse results in intense pruritus and generalized scaling and crusting with excoriations and lichenification. A secondary skin infection may result from the pruritus. One may elicit the itch-scratch reflex by scratching the horse over the withers, causing the horse to tuck its nose close to its chest and make smacking noises and exaggerated lip movements. This reflex suggests scabies but is not diagnostic, and one may elicit the reflex in some normal horses.14 The scabies mite is circular with short legs, a terminal anus, and long unjointed pedicles. Unfortunately, as in other species, the scabies mites are difficult to find on skin scrapings. Therefore a negative skin scraping does not ensure that the horse does not have scabies.
Chorioptes equi
Chorioptes equi mites are host-specific and do not parasitize human beings (Figure 13-4). Unlike the scabies mite, infestation with C. equi is not a reportable condition. C. equi mites spend their entire life cycle on the host feeding on epidermal debris. Preferred feeding sites include the distal limbs and perineum. Infested horses exhibit intense pruritus with stomping of feet and rubbing of the perineal area. Skin lesions include alopecia, erythema, and crusting of the pasterns, fetlocks, or perineum. Draft-breed horses or other horses with feathered fetlocks are predisposed to C. equi. The mite tends to be more common in the winter months. C. equi should be included in the list of differential diagnoses for horses with pastern dermatitis or greasy heel. Treatment of this mite is the same as for S. scabiei. However, ivermectin-resistant C. equi mites have been reported, and topical dip therapy may be indicated if the horse does not respond to oral therapy. If a topical medication is used, clipping the hair around the fetlocks and pasterns to ensure optimal contact of the dip with the skin and mites is advisable.
Trombiculidiasis
Trombiculidiasis (chiggers, red bugs, harvest mites) is caused by an infestation with larvae of free-living adult mites (genus Eutrombicula or Neotrombicula). Larvae are most prevalent in grasses, forests, or swamps in late summer and fall. Small rodents are the natural host. Pathognomonic skin lesions are papules or wheals with a small orange or red dot (trombiculid larvae) in the center. Trombiculidiasis is seasonal, but the seasonality varies with genus and species. Eutrombicula alfreddugesi is active in the late spring and peaks midsummer, whereas Neotrombicula automnalis is most active from the late summer to midautumn.10 Larvae feed on the host in the late afternoon and early evening. Areas of the body most commonly infested are the face, muzzle, distal limbs, ventral thorax, and abdomen. The diagnosis is based on observation of the distinctive lesion with the larvae approximately 0.2 to 0.4 mm in size, oval in shape with six legs, in the center of the skin lesion. Trombiculidiasis is a self-limiting disease. Some horses are uncomfortable with this mite infestation and should be treated. Recommended treatments include lime sulfur dip, permethrin, pyrethrin, cypermethrin, and phoxim sprays or dips as a one-time treatment in association with prednisolone at a dose of 0.5 mg/kg orally for 3 to 5 days.
Pyemotes tritici
Pyemotes tritici (straw itch mite) normally parasitizes the larvae of grain insects. Occasionally this mite parasitizes human beings and horses.15 Infestation in horses occurs from contaminated hay fed in overhead racks. The mite produces a maculopapular crusted eruption on the head, neck, and trunk that is only occasionally pruritic. The diagnosis is based on history and clinical examination. This disease is self-limiting, and the owner should remove contaminated forage or feed the horse from the ground until all contaminated forage has been consumed. Infestations from fomites or hay fed on the ground have not been reported.
Dermanyssus gallinae
This mite causes pruritic papules and crust of the head and legs of horses. Skin scrapings and tape preparations at night yield the best chance of obtaining the mite. Routine mite treatments that were discussed previously are also beneficial for treating this mite. However, with the poultry mite, one needs to treat the environment and remove the bird nests from the stable or eliminate the horse’s contact with poultry.10
Demodicosis
Demodicosis is a rare equine skin condition. Two species of Demodex can infest horses (i.e., D. equi and D. caballi), and each affects different areas of the body. D. equi affects the body, whereas D. caballi prefers the eyelids and muzzle. Clinically these horses have one or more alopecic or scaling areas of the body. The most common areas of the body affected in horses with demodex are the face, neck, and sometimes shoulders. The diagnosis is based on skin scrapings. Demodex mites are easily found on skin scrapings. Several different treatment options exist for treating equine demodex. Treatment with 2% trichlorfon topically every other day has been used with success. Daily ivermectin or dormectin has also been used with some success. Amitraz is contraindicated because this drug causes colic in horses. Regardless of the treatment used, one needs to identify and treat the underlying cause for the demodicosis. The most common underlying causes for equine demodicosis are chronic glucocorticoid use and equine Cushing’s disease (pituitary pars intermedia dysfunction).10,12
Ticks
Dermacentor, Ixodes, and Amblyomma species are the most common ixodid ticks of horses. These ticks are found outdoors and have complicated life cycles in which all stages of the life cycle are parasitic. The severity of clinical signs depends on the density of the infestation and whether the horse develops a hypersensitivity reaction to the bites. Infestations occur most commonly on the ears, face, neck, groin, distal limbs, and tail. Early lesions consist of papular to pustular eruptions that rapidly develop into crusts, erosions, ulcers, and hair loss. Hypersensitivity reactions may be local or general. Local responses appear as nodules that develop at the site of the tick bite. Although the pathogenesis is unknown, cutaneous basophil hypersensitivity is believed to be involved.16 Systemic reactions are characterized by whole-body urticaria or multifocal urticarial plaques. In Australia, a hypersensitivity reaction to Boophilus microplus has been observed in sensitized horses.17 Intense pruritus, papules, and wheals develop as soon as 30 minutes after ticks begin to feed.
A definitive diagnosis is made by observation of ticks attached to the horse or in the ear canal. Treatment aims at killing ticks on the horse. The practitioner should apply pyrethrin or pyrethroid sponge-on dips to the body of the horse, taking extra care to soak skinfold areas. Usually, only one treatment is necessary unless reinfestation occurs. Resistance to insecticides can occur rapidly, and knowledge of local resistance patterns is important. Infestations of O. megnini require mechanical removal of as many ticks as possible. One part rotenone and three parts mineral oil applied twice weekly is an effective otic parasiticide for horses. The author has used a commercially available pyrethrin otic preparation (Otomite Plus, Vibac, Fort Worth, Tex.) for small animals in horse ears with success. Ivermectin also has been shown to be effective in treating ticks at a dose of 200 μg/kg orally.
Onchocerciasis
Onchocerciasis is a nonseasonal skin disease of the horse caused by the parasite Onchocerca cervicalis. This nematode lives in the ligamentum nuchae and produces microfilariae that migrate to the skin and are ingested by the intermediate host, Culicoides species. Microfilarial populations in the skin vary, and the highest concentrations occur in the dermis of the face, neck, and ventral midline, especially the umbilicus (Figure 13-5).18 Microfilarial populations vary seasonally and are highest in the spring, which interestingly is the peak season for the Culicoides vector.19 Microfilariae are more superficial in the dermis during the spring and summer months. Clinical signs of onchocerciasis are believed to be caused by an idiosyncratic hypersensitivity reaction to one or more microfilarial antigens because many horses that have circulating microfilariae do not have any gross skin or ocular lesions.20 Whether the reaction is directed only at dying or dead microfilariae is unknown.
Onchocerciasis has no breed or sex predilection and usually affects horses 4 years of age and older. Clinical signs are nonseasonal but may be worse in the spring and summer, most likely because of the added irritation from the vector. Lesions may occur on the face and neck, on the ventral chest and abdomen, or in all these areas.21,22 Early lesions begin as a thinning hair coat. As the disease progresses, lesions may vary from focal to generalized areas of alopecia, scaling, crusting, and plaques. Affected areas may be severely excoriated, ulcerated, oozing, and lichenified. Annular lesions in the center of the forehead suggest the disease (Figure 13-6). Leukoderma usually develops at the site of lesions and is irreversible. Ocular lesions include sclerosing keratitis, vitiligo of the bulbar conjunctiva, white nodules in the pigmented conjunctiva, uveitis, and a crescent-shaped patch of depigmentation bordering the optic disk.23
The practitioner may make a presumptive diagnosis of onchocerciasis by demonstrating the microfilariae in the skin of animals with compatible historical and clinical findings. Skin scrapings and direct blood smears are often negative. One can demonstrate microfilariae most reliably with a mince preparation or via histologic examination of skin from a biopsy specimen.22 Mince preparations require a 4- or 6-mm punch specimen of tissue obtained from the ventral abdomen. One places the tissue specimen in a Petri dish with a small amount of physiologic saline, minces it with a scalpel blade or razor, and incubates it at room temperature for 30 to 60 minutes. The specimen can then be examined microscopically for evidence of the rapid motion of the microfilariae. Skin biopsies reveal a superficial perivascular eosinophilic dermatitis. Often, microfilariae are visible in the superficial dermis.7
Ivermectin at a dose of 200 μg/kg orally is the treatment of choice.7,21,24 A single dose often produces remission of clinical signs within 2 to 3 weeks. However, some horses require two to three monthly treatments before clinical signs resolve. Approximately 25% of horses have an adverse reaction, such as ventral midline edema or pruritus, which occurs 1 to 10 days after treatment. In rare cases, severe umbilical and eyelid edema and fever may occur. In confirmed cases of onchocerciasis, one should perform a thorough ocular examination to look for any signs of eye disease associated with onchocerciasis. Anecdotal reports suggest treatment may precipitate an episode of uveitis.
Habronemiasis
A horse with cutaneous habronemiasis has ulcerative nodules in the spring and summer that partially or completely regress in the winter. A recent study suggests that Arabians, gray horses, and horses with diluted color (i.e., palomino, buckskin, dun) may be predisposed to developing cutaneous habronemiasis.25 Interestingly, in this same study, Thoroughbred horses were the most underrespresented breed of horse for cutaneous habronemiais. No breed, sex, or age predilection exists. Some horses may be predisposed to cutaneous habronemiasis, exhibiting clinical signs each year, whereas other horses on the same premises never develop this condition.
Lesions often are ulcerated and appear similar to exuberant granulation tissue. Yellow granules approximately 1 mm in diameter may be present.26,27 Microscopic examination of these granules does not reveal branching hyphae such as one frequently observes with granules from pythiosis or zygomycosis lesions.
The diagnosis is based on history, physical examination, cytologic examination, and biopsy of lesions. Cytologic examination of exudate sometimes reveals the presence of nematode larvae. These larvae are large (3 mm by 60 μm), with a spiny tail, and are usually motile. Skin biopsies reveal a nodular to diffuse granulomatous dermatitis with large numbers of mast cells and eosinophils. Foci of coagulation necrosis are characteristic.28,29 Sometimes this area of necrosis contains cross-sections of larvae.
Treatment of cutaneous habronemiasis should include control of the associated hypersensitivity reaction and elimination of the parasite. Treating horses with ivermectin alone is not always effective. The author and co-workers examined 14 horses between 1988 and 1998 that had been treated with ivermectin in the preceding month and had intralesional habronema larvae present on biopsy. Similarly, 5 of 31 horses treated with ivermectin for habronemiasis required an additional treatment 2 to 4 weeks later.30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree