CHAPTER 9 Disorders of the Respiratory System
Disorders of the respiratory system are second in importance only to those of the musculoskeletal system in limiting the athletic performance of the horse. Early detection and treatment of respiratory problems is essential to reduce economic losses and to prevent premature retirement of the horse from athletic performance.
DIAGNOSTIC APPROACH TO RESPIRATORY DISORDERS
History
AGE AND BREED
Breed considerations are also important in investigating respiratory disorders. For example, one should evaluate young Arabian foals with chronic infections for combined immunodeficiency syndrome, a heritable condition in which cell-mediated and humoral limbs of the immune system are deficient. In addition, solitary defects in the humoral immune system also predispose horses to develop chronic respiratory and enteric infections. Selective immunoglobulin M (IgM) deficiency tends to occur more frequently in Arabians and Quarter Horses than in other breeds, whereas agammaglobulinemia has been documented in Thoroughbreds and Standardbreds.1,2
PRESENT MEDICAL PROBLEM
Questions should be focused on defining the exact problem, establishing the chronicity of the disorder and the rapidity of its development. Is the problem insidious in onset or an acute disorder of less than 2 weeks’ duration? Is the onset associated with a stressful event such as racing or a prolonged van ride? Does the farm have new arrivals that have not been quarantined? One should also determine the effect of the respiratory complaint on the expected athletic performance: Are clinical signs evident during eupneic (resting) breathing, or are they noticeable only during the hyperpnea of exercise? Has the horse received any medication, and did the clinical condition improve? Amelioration of signs during therapy suggests that the previous treatment protocols were not of sufficient duration or that an underlying immunodeficiency exists.
Physical Examination
Before physically examining the horse, it is helpful to simply step back and observe the demeanor and mental status (alert or depressed), posture, and manner of movement of the horse. Has the horse adopted a particular stance (extended head and neck), or is it reluctant to move because of pain (pleurodynia)? Are changes in the pattern of breathing from the normal eupneic state obvious (Box 9-1)? Is the breathing pattern rapid and shallow? Does nostril flaring accompany a pronounced expiratory effort? The normal respiratory rate of the adult horse varies from 8 to 15 breaths per minute, with a slightly noticeable abdominal component during expiration (an active process in the horse).
BOX 9-1 BREATHING PATTERNS
Tachypnea
A breathing pattern characterized by rapid frequency and shallow depth or small tidal volume.
Hyperventilation
A pattern of breathing that increases alveolar ventilation and results in arterial hypocapnia.
Percussion of the frontal and maxillary sinuses, performed by gently tapping over the sinuses while one holds the mouth of the horse slightly open, may reveal a dullness because of accumulations of fluid or inflammatory products. The absence of a percussible change does not rule out a sinus disorder. One also should assess evidence of swelling in the submandibular space (lymphadenopathy) or in the pharyngeal (guttural pouch or retropharyngeal disorders) and cervical areas (presence of accessory lungs).3 Palpation of the laryngeal cartilages is important. Notably, the palpation of a more prominent muscular process of the arytenoid cartilage, caused by dorsal cricoarytenoid muscle atrophy, is subjective and may not be a good predictor of the presence of recurrent laryngeal neuropathy (RLN). A surgical scar can be detected by picking up and rolling the skin at the potential surgical site between the thumb and index finger. These sites include the dorsal lateral larynx (laryngoplasty), the intermandibular space in the midline and level with the vertical rami of the mandibles (laryngeal tie-forward) and cricothyroid notch (arytenoidectomy, vocal cordectomy). Any disparity in the angle of the lashes of the upper eyelids should also be noted. Vertical eyelashes and eyelid drooping in one eye (ptosis as a result of Horner’s syndrome) suggest dysfunction of Müller muscle caused by damage of the vagosympathetic trunk.4,5 Tracheal palpation is routine and should not elicit coughing episodes in the normal horse. The patency of the jugular veins should also be checked. Any evidence of perivascular injections that may contribute to upper respiratory tract obstructions by involving the recurrent laryngeal nerve or vagosympathetic trunks should be noted.
AUSCULTATION OF THE LUNG FIELDS
Examine the horse during eupneic and hyperpneic (by use of a rebreathing bag) breathing. Normal breath sounds are those produced by turbulent air movement through the tracheobronchial tree and vary in intensity and quality depending on the portion of the lung field auscultated. The vesicular sounds, over the middle and diaphragmatic lung lobes, are the quietest sounds; the bronchial sounds, over the trachea and the base of the lung, are the loudest.6–8 In the normal horse, one can hear breath sounds more easily on the right side than on the left. Considerable variation exists between normal patients in the intensity of the breath sounds. For example, vesicular sounds are often barely audible during eupneic (normal) breathing in the obese patient and are perceived as soft rustling sounds. However, one may hear breath sounds easily in the thin or young animal because of less attenuation of lung sounds by the chest wall. The intensity of breath sounds also increases with increased airflow. Thus breath sounds are accentuated in febrile or excited animals or in animals hyperpneic from a variety of causes (exercise, hypoxia, pain). However, auscultatory findings do not always correlate well with the degree of alveolar ventilation.7 For example, in horses with lung consolidation, the transmission of breath sounds from adjacent areas gives the false impression that that region is well ventilated. Breath sounds also may become more difficult to hear in cases of (1) alveolar overinflation in which the aerated tissue of the lung is a poor conduction medium of sound or (2) pneumothorax and pleural effusions in which the sound is reflected at the pleural surface (acoustic impedance).7
Adventitious lung sounds are abnormal sounds superimposed on the normal breath sounds and have been described as crackles or wheezes. Crackles are short, explosive, discontinuous sounds that have been likened to the sound of salt thrown in a hot frying pan or the sound of cellophane being crumpled. They are usually of low intensity and are audible during the inspiratory phase of respiration. Their production has been attributed to the sudden equalization of pressure in two compartments after airways have reopened. Crackles are often audible in horses with pneumonia, pulmonary edema, or recurrent airway obstruction. Breathing 100% oxygen also may produce crackles because the nitrogen stent maintaining alveolar distention is eliminated.9 Crackles are also audible in horses with subcutaneous emphysema. Wheezes are musical sounds thought to arise from the vibration of airway walls or tissue masses in close contact with the airway walls and may be audible during inspiration or expiration. Wheezes may be monophonic or polyphonic, the latter indicative of multiple sites of airway obstruction. Pleuritic friction rubs have been described as sandpaper-like sounds generated by the movement of the visceral and parietal pleurae across each other. One may detect them (infrequently) in the early stages of dry pleuritis, before the effusive stage.
PERCUSSION OF THE THORAX
Percussion of the thorax is accomplished by evaluating the type of sounds produced as one systematically taps the intercostal spaces of the thorax using a plexor and pleximeter (foals) or a large spoon and neurologic hammer (adults). Alternatively, an Azary pleximeter that is made of horn and a metal percussion hammer with a rubber tip may be used because of its improved sound quality.10 Aerated tissues produce a resonant sound, whereas fluid-filled structures (bowel, heart, lung abscesses, consolidated lung) produce a dull sound. One should identify the transitional site where the sound quality changes during percussion (the site is marked with a piece of tape) and then compare with the normal limits in a healthy horse. The cranial limit is the shoulder musculature, the dorsal limit is the back musculature, and the caudoventral limits are the seventeenth intercostal space at the level of the tuber coxae, the sixteenth intercostal space at the level of the tuber ischii, the thirteenth intercostal space at the mid thorax, the eleventh intercostal space at the level of the scapulohumeral articulation, and the sixth intercostal space at the olecranon.11 Defining the caudal lung borders of the lung field by thoracic percussion, at least in healthy horses, agrees well with the more routinely used method of ultrasonography.10 Occasionally, the gas content of the large colon on the left side or of the cecum on the right side precludes detection of the caudal lung border in the sixteenth intercostal space in some horses.10 Thoracic percussion should not be painful; resentment by the horse may indicate pleuritis or rib fractures. A ventral dullness suggests pleural effusion, pleural thickening, lung consolidation, masses, or pericardial effusion. Occasionally, caudal borders may be expanded, suggesting alveolar overinflation, which may accompany recurrent airway disease.
ENDOSCOPY
Fiberoptic endoscopy is an essential tool for assessing the equine respiratory tract. Endoscopy is helpful in establishing (1) the origin of respiratory noises that accompany laryngeal hemiplegia, dorsal displacement of the soft palate, epiglottic entrapment, rostral displacement of the palatopharyngeal arches, arytenoid chondritis, tracheal collapse or stenosis, and pharyngeal narrowing; (2) the existence of congenital defects such as subepiglottic cysts, cleft palate, or choanal atresia; and (3) the source of exudate or hemorrhage occurring with guttural pouch mycosis or empyema, ethmoidal hematoma, pulmonary epistaxis, retropharyngeal abscessation, and chronic pulmonary disease. Endoscopy also enables one to extract foreign bodies from the tracheobronchial tree and to obtain biopsies of tissues or masses in the respiratory tract. Endoscopic visualization of exudates within the pharynx or the trachea after exercise suggests the presence of inflammatory airway disease. Endoscopy is also used to obtain tracheobronchial aspirates (guarded swabs or catheters)12 or to perform bronchoalveolar lavage (see later discussion). Several different fiberoptic endoscope models are available, but an 11-mm (outer diameter) endoscope is usually used in adult horses, and a smaller, 7.8-mm (outer diameter) pediatric scope is recommended for foals.
Videoendoscopic examination of the upper respiratory tract during maximal treadmill exercise (12 to 14 m/s) is a routine diagnostic modality at many referral centers, allowing visualization of dynamic collapse of the upper airway structures during exercise. Several studies have described the presence of multiple sources of airway collapse at high speed, including the aryepiglottic folds, vocal cords, and arytenoid cartilages.13–15 Frequently, an abnormality in one structure produces narrowing of the airway, making inspiratory pressures more negative and leading to collapse of other structures. In one study, more than one abnormality was identified in 49% of the horses.15 Accurate identification and appropriate treatment of these complex cases is not possible without a treadmill examination.
SINUSCOPY
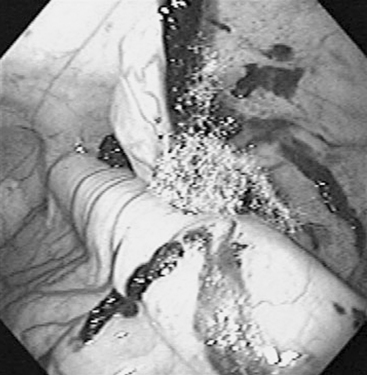
Sinuscopy entails direct examination of the interior of the paranasal sinuses using an endoscope or an arthroscope that is introduced through a small trephine opening and is useful for diagnosing or treating sinus disease.16,17 In selected cases, sinuscopy may be a diagnostic or therapeutic alternative to flap sinusotomy. The caudal maxillary sinus can be approached at a point 2 cm rostral to the midpoint of a perpendicular line drawn from the medial canthus of the eye to the facial crest. The rostral maxillary sinus can be accessed 2 cm caudal to the midpoint of a line drawn from the rostral end of the facial crest to the infraorbital foramen. The frontoconchal sinus is accessible through a portal placed 40% of the distance of a perpendicular line drawn from 0.5 cm caudal to the medial canthus from the midline. The site affords excellent visualization of the frontoconchal sinus and, via the large frontomaxillary opening, the caudal maxillary sinus. After sedation of the horse and surgical preparation and local anesthesia of the selected site, the clinician makes a small skin incision and opens the bone with either a 0.25-inch Steinmann pin (for a 4-mm diameter arthroscope) or a 10-mm trephine (for a 9-mm endoscope). The endoscope provides better illumination and a greater field of view. In addition to direct examination of the sinus interior for diagnosis (Figure 9-1), sinuscopy allows the examiner to biopsy tissues under direct visualization and to apply therapeutic procedures such as formalin injection of mass lesions (Figure 9-2), cyst removal, and removal of sequestered bone fragments. After the examination, the clinician may close the portal with cutaneous sutures or leave it open for therapeutic lavage.
COMPUTED TOMOGRAPHY
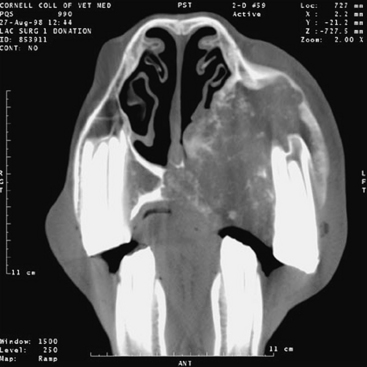
Interpretation of conventional radiographs of the equine head is notoriously difficult because of the complex anatomy and the spectrum of radiographic densities: air, soft tissue, bone, and tooth. Radiographs and clinical examination may not define accurately the location and extent of lesions in the head region, necessitating computed tomographic (CT) scans.18 CT uses a rotating, highly columnated x-ray beam to generate digital cross-sectional images.19,20 These cross-sectional images afford a much better evaluation of normal and pathologic anatomy than can be achieved through conventional radiographs (Figures 9-3 to 9-5). Additionally, the digital format enables three-dimensional reconstruction of structures and manipulation of images to optimize interpretation (see Figure 9-4). Constraints for this technique are its expense and limited availability and the need for general anesthesia of the horse.
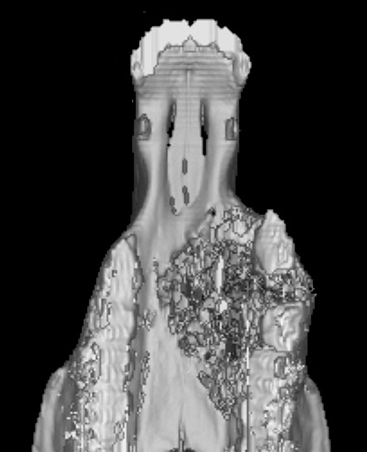
FIGURE 9-4 Three-dimensional reconstruction of the premaxilla in Figure 9-3. The extent of osseous destruction of the hard palate can be delineated clearly, as can separation of the second and third premolars.
(Courtesy R. P. Hackett, Ithaca, N.Y., 2001.)
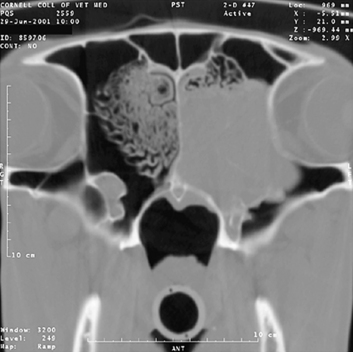
FIGURE 9-5 Cross-sectional image of an adenocarcinoma affecting the ethmoid turbinate in a 23-year-old hinny.
(Courtesy R. P. Hackett, Ithaca, N.Y., 2001.)
Computed tomography has proved particularly useful for examining the anatomically complicated structures of nasal turbinates, paranasal sinuses, teeth, nasopharynx, and guttural pouches and for evaluating areas obscured by overlap of adjacent structures on conventional radiographs.18,21–25 CT is better than plain radiography for the diagnosis of dental disease caused by inherent avoidance of superimposition of the opposite dental arcade, excellent bone density characterization, and good spatial resolution.26 Improved evaluation of these structures has enhanced considerably the clinician’s ability to determine an accurate diagnosis, select optimal surgical approaches, and render an appropriate prognosis.
Computed tomography has been of limited usefulness in evaluating the lower respiratory tract in adult horses because of the size constraints of the gantry. However, CT is an excellent diagnostic modality for detecting and defining the extent of pulmonary or mediastinal masses in the thorax of foals, ponies, and miniature horses.27
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is another modality that can be used to investigate upper respiratory tract disorders. Magnetic resonance images represent a map of tissue protons that, under the influence of a magnetic field, are excited by brief pulses of radiofrequency waves.19 As the magnetized excited protons relax back to their original magnetized state, a signal is emitted, the intensity of which is converted to a gray-scale image. MRI provides excellent cross-sectional evaluations of soft tissue structures, although it also provides useful information about bony structures. Contrast enhancement can be achieved by injecting an intravenous derivative of gadolinium during the T1-weighted scans.19 Magnetic resonance images of the equine sinuses and nasal passages have been previously described,28 although these images were obtained from cadaver heads. More recently, a detailed MRI study of horses with neurologic signs localized to the cerebrum, brainstem, or both was published demonstrating that equine skull structures can be imaged.29 The authors note that compared with MRI in small animals, this imaging technique requires additional modifications with respect to the use of the contrast agent (gadolinium-diethylene triamine pentaacetic acid dose) and the scanning room, which must accommodate an anesthetized horse positioned on a nonmetallic support table. Because of equipment size constraints, MRI would likely be used only to evaluate thoracic structures in foals or miniature horses.
SAMPLING OF RESPIRATORY TRACT SECRETIONS
Guttural Pouch Catheterization and Culture of the Exudate
One performs guttural pouch catheterization in cases of empyema, chondroids, or distention of the pouches or in suspected cases of S. equi subsp. equi infections. With the horse sedated, the clinician passes a fiberoptic endoscope into the guttural pouch using the biopsy instrument or a Chambers catheter as a guide for the endoscope. One obtains a sample of the exudate with a triple-guarded catheter or protected swab. A technique for obtaining percutaneous aspirates of the guttural pouches recently has been described, but the authors prefer to use endoscopic visualization for sample collection.30 Cytologic examination of guttural pouch aspirates from normal horses demonstrates the presence of mucus, a predominance of ciliated columnar epithelial cells, neutrophils, and a few (<1%) macrophages, lymphocytes, and eosinophils. Aspirates are not normally sterile; common bacterial isolates include β-hemolytic Streptococcus, Staphylococcus, and Moraxella species.31 S. equi subsp. equi is not a normal inhabitant of the guttural pouch.
Sampling of Tracheobronchial Secretions
Several techniques have been advocated for obtaining tracheobronchial samples. The choice of site for sample collection (tracheal versus bronchoalveolar) depends on the nature of the respiratory disorder. The appropriateness of using tracheal samples to evaluate chronic inflammatory disorders has been challenged because little correlation exists between tracheal and bronchoalveolar lavage cytologic findings or between tracheal and pulmonary histopathologic findings.32,33 In contrast, a good correlation exists between bronchoalveolar lavage cytologic findings and pulmonary histopathologic findings.34
Tracheobronchial aspirates remain the method of choice for the investigation of infectious lower respiratory tract disorders. Collection of culture samples by fiberoptic endoscopy simplifies the procedure and eliminates some of the complications formerly associated with the transtracheal technique, such as cellulitis and pneumomediastinum. Guarded tracheal swabs35 and the telescoping plugged catheter of Darien12 are convenient techniques for obtaining representative samples. However, oropharyngeal contamination may still occur when one obtains tracheobronchial aspirates endoscopically using telescoping plugged catheters.36,37 The authors prefer to obtain tracheobronchial aspirates percutaneously.
Characterization of the normal bacterial isolates from tracheobronchial aspirates in healthy horses has been well documented. When examining a horse with suspected respiratory disease, one must evaluate culture results in light of the cytologic findings and clinical examination. The tracheobronchial aspirates of approximately 8% of normal horses (pastured or stabled) were found to be culture positive for Klebsiella, β-hemolytic streptococci, Pasteurella species, and Pseudomonas aeruginosa. For a microorganism to be implicated in a lower airway disorder, one would expect (1) to obtain a moderate to heavy growth of the organism on culture (≥1 × 103 colony-forming units), (2) to identify organisms within phagocytic cells, and (3) to have evidence of degenerative neutrophils. In contrast, anaerobes, which are a normal component of the oropharyngeal flora, are normally not isolated from aspirates of healthy horses, emphasizing their importance in disease processes when recovered from respiratory cases. Based on their studies, Sweeney, Beech, and Roby38 also have described a group of transient bacterial flora of questionable pathogenicity such as Enterobacter, Bacillus, Acinetobacter, β-hemolytic streptococci (except for Streptococcus pneumoniae type 3), and Staphylococcus epidermidis, which may be isolated from tracheobronchial aspirates. In contrast, S. pneumoniae is now recognized as a pathogen of the respiratory tract in horses.39–41 Fungal hyphae may be found free or engulfed within mononuclear cells in normal horses.
Bronchoalveolar Lavage
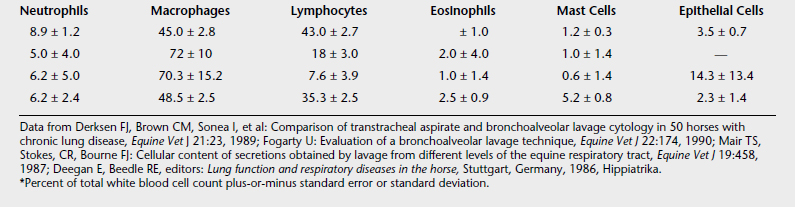
This procedure is indicated in the investigation of chronic inflammatory diseases but may be performed with collection of transtracheal aspirates if one cannot dismiss an infectious process. Table 9-1 shows representative cytologic findings from bronchoalveolar lavage studies of normal horses. Before performing the bronchoalveolar lavage, the horse is sedated. Either a fiberoptic endoscope, which permits direct visualization of the lung segment to be lavaged, or a thick-walled flexible tube with a cuffed end, which is passed blindly into the distal airways, is wedged into the distal airway. Then, 20 to 35 ml of 2% lidocaine may be infused into the airways to desensitize the lung segment being sampled. Approximately 60 (foal) to 300 ml (adult horse) of physiologic saline solution is instilled and then aspirated. In healthy horses, nearly 75% of the fluid can be retrieved for cytologic examination. This volume is more difficult to obtain in horses with airway inflammation and mucus plugs.
Radiography
This technique may be helpful (1) in detecting soft tissue masses (abscesses, granulomata, neoplasms, hematomas, polyps) or fluid accumulations within the paranasal sinuses, the nasal cavity proper, the guttural pouches, and the retropharyngeal areas and (2) in evaluating orofacial deformations or fractures after trauma. Radiography also allows assessment of the anatomic dimensions of the pharyngeal and laryngeal structures (thickened soft palate, hypoplastic epiglottis, hyoid bone fractures). When nasal or sinus disorders are suspected, one should take lateral, dorsoventral, and oblique views. The clinician can usually achieve proper restraint of the horse for positioning of the cassette with xylazine, detomidine, or butorphanol sedation. In the horse that can be anesthetized safely, one may obtain a more thorough definition of the extent of nasal and upper respiratory tract diseases using CT scans.
Radiographic evaluation of the equine thorax remains preferable to ultrasonography for detecting diffuse parenchymal diseases such as interstitial pneumonia, pulmonary edema, and chronic airway disorders and for detecting deep parenchymal abscesses.42 Imaging the thorax of the standing horse requires three to four overlapping lateral radiographs. However, compared with human or small animal medicine, in which correlations between the pulmonary disorders and the radiographic findings are well established, the radiographic changes in equine respiratory disorders tend to be rather nonspecific and lack predictive value in inflammatory airway diseases.43 In addition, many pulmonary diseases such as inflammatory airway disease, exercise-induced pulmonary hemorrhage, lungworm infections, and recurrent airway obstruction may not have detectable radiographic abnormalities.43
Four types of radiographic patterns have been described: alveolar (airspace), interstitial, bronchial, and vascular. In the alveolar pattern, opaque areas coalesce and completely obliterate the vessels and bronchi. Air bronchograms may be notable. This pattern occurs with pneumonia, pulmonary edema, hemorrhage, or neoplastic infiltration. Interstitial patterns are found most commonly and are associated with a variety of conditions. This pattern causes a blurring of the edges of the pulmonary vessels, a diffuse increase in lung opacity, and variable reticular, linear, or nodular opacities.43,44 A reticular pattern occurs with viral, bacterial, or parasitic pneumonia; pulmonary edema; interstitial pneumonia; and pulmonary fibrosis. An irregular linear pattern occurs with resolving bronchopneumonia, and a nodular pattern occurs with abscesses, granulomata, or neoplasms. Bronchial patterns alone are not found commonly but usually occur in association with interstitial patterns. Paired linear opacities or numerous small circular opacities represent thickening of the large- and medium-sized airways or of the septa around the lobules. This pattern occurs in cases of equine bronchitis. Bronchiolitis is generally not recognized radiographically.45 Variations in the size, shape, and number of the pulmonary vessels cause a vascular pattern and may be visible in horses after exercise or in horses with left-to-right cardiac shunts.
Ultrasonography
Thoracic ultrasonography is useful for diagnostic, therapeutic, and prognostic evaluation of peripheral parenchymal lung or pleural disorders. Unlike thoracic radiography, which requires technology limited to specialty practices or veterinary medical teaching hospitals, ultrasonography is a method readily available to the practicing veterinarian. Ultrasonography is considered to be superior to thoracic radiography for detecting pleural effusion, pulmonary consolidation, pulmonary or mediastinal abscesses, tumors, or granulomata42 and should be performed when clinical examination or thoracic percussion reveals pain and areas of dullness within the thorax.
Normal lung tissue reflects the ultrasound beam, producing an echogenic pulmonary periphery (thin white line) and reverberation artifacts or concentric equidistant echoes. Normal pleural fluid appears as an anechoic (black) area separating the parietal pleura from the lung tissue, and one commonly detects a small amount of pleural fluid in the ventral thorax of racehorses.46 In respiratory disorders, one may detect accentuated amounts of anechoic or hypoechoic (gray) pleural fluid. The clinician can determine the character of the pleural fluid, the presence of fibrin or gas, the degree of loculation, and the existence of pleural adhesions during the examination. Pulmonary abscesses appear on ultrasonography as encapsulated cavitated areas filled with fluid or echogenic (white) material, whereas areas of pulmonary consolidation appear as dense patterns of homogeneous internal echoes with a gray tone.47 Depending on the degree of consolidation, one may visualize bronchial and vascular structures more easily on the sonogram, as well as mediastinal masses. Detection of caudal mediastinal masses improves when pleural effusion is concurrent, because the aerated caudal lungs impair examination. One may visualize masses located in the cranial mediastinum at the third right intercostal space in the absence of pleural effusions.
Diagnostically, certain limitations are inherent in ultrasonography. One may not detect a deep parenchymal lesion if the overlying aerated lung reflects the ultrasound beam. In addition, cases of pneumothorax may be difficult to identify because the free air in the dorsal thorax and the aerated ventral lung appear similar with ultrasound.42 One also may use ultrasonography prognostically. The detection of free gas echoes (associated with anaerobic bacterial infections or bronchopleural fistulae), extensive fibrinous tags, or areas of loculations within the pleural fluid are associated with a poor prognosis, requiring a more extensive therapeutic regimen.48
Thoracocentesis
Sampling of the pleural fluid by means of thoracocentesis is beneficial for diagnostic, prognostic, and therapeutic purposes. Abnormal pleural fluid accompanies numerous respiratory disorders, including pulmonary abscessation (pleuropneumonia), chronic pneumonia, systemic mycoses, neoplasia, pulmonary granulomata, and equine infectious anemia. One may perform the technique easily at the sixth or seventh intercostal space approximately 10 cm dorsal to the olecranon by aseptically inserting a teat cannula through an anesthetized site in the intercostal space, just cranial to the border of the rib. To reduce the amount of air aspirated into the pleural cavity, one attaches a three-way stopcock to the cannula. The clinician should take samples from both sides of the thorax and submit the aspirate for cytologic and microbiologic examination. One may obtain up to 100 ml of pleural fluid, although smaller amounts (10 to 30 ml) are more routine.49 Normal pleural aspirates contain less than 10,000 nucleated cells per μl (60% of which are neutrophils) and less than 2.5 g/dl of protein. Samples should be cultured aerobically and anaerobically. Fluid with a putrid odor is associated with anaerobic bacteria and carries a less-favorable prognosis for the horse.50
Nuclear Medicine Imaging
Scintigraphy, or nuclear medicine imaging, is a specialized technique available at some university and practice facilities. Using gamma-emitting radioisotopes such as krypton-81m or technetium-99m, the clinician can assess pulmonary ventilation and perfusion in the horse.51,52 The horse, breathing through a closed circuit, inhales aerosolized technetium particles generated by a nebulizer. Aerosolized particles are of sufficiently small diameter to be deposited in the alveoli and small airways. Thus their distribution mirrors ventilation. A gamma camera records the sites of deposition within the lung fields. For the perfusion scan, one injects technetium-labeled macroaggregated albumin intravenously. The large protein particles lodge in the blood capillaries of the lung, enabling imaging of the pulmonary perfusion by the gamma camera. Hence lung scintigraphy permits evaluation of the ratio of regional ventilation to perfusion (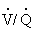 ) not possible by radiography or ultrasonography. Scintigraphic images may provide additional insights into the diagnosis and pathogenesis of such disorders as recurrent airway obstruction or exercise-induced pulmonary hemorrhage (EIPH) or in the evaluation of the horse with poor performance.53,54 For example, horses with EIPH appear to have a perfusion deficit in the caudodorsal lung lobe that results in a high (
) not possible by radiography or ultrasonography. Scintigraphic images may provide additional insights into the diagnosis and pathogenesis of such disorders as recurrent airway obstruction or exercise-induced pulmonary hemorrhage (EIPH) or in the evaluation of the horse with poor performance.53,54 For example, horses with EIPH appear to have a perfusion deficit in the caudodorsal lung lobe that results in a high (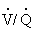 ) area.53 Horses with recurrent airway obstruction may produce several patterns, including (
) area.53 Horses with recurrent airway obstruction may produce several patterns, including (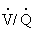 ) deficits in the costophrenic angle (caudoventral diaphragmatic margin), ventilation deficits in the mid dorsal lung area, or patterns similar to those seen in EIPH.55
) deficits in the costophrenic angle (caudoventral diaphragmatic margin), ventilation deficits in the mid dorsal lung area, or patterns similar to those seen in EIPH.55
In addition to assessment of pulmonary ventilation and perfusion, scintigraphy can show tracheal mucus transport after intratracheal injection of technetium. One performs the technique by timing the movement of the radioactive bolus over a given tracheal distance.56,57 Normal values for the unsedated horse range between 16.6 to 20.7 mm/min. Further studies are needed to examine alterations in tracheal mucus transport during disease. For further information, the reader is referred to additional reviews.56 Nuclear scintigraphy is not more useful than radiographs for the assessment of sinus disorders.58
Pulmonary Function Testing
Measurements of mechanical properties of the respiratory system, airway hyperreactivities, and arterial blood gas determinations have been used to assess the lower respiratory tract in horses and are generally available only at specialized pulmonary units associated with referral centers. The conventional techniques of pulmonary function testing require that (1) the horse wear a breathing apparatus to which an airflow meter has been attached and that (2) pleural pressure changes are measured by a catheter placed in the mid-esophagus, exteriorized through the nares, and attached to a pressure transducer. By integrating airflow relative to time, one obtains the inspiratory and expiratory volumes. Additional parameters obtained from measurements of airflow include inspiratory and expiratory times, breathing frequency, and peak airflows. In general, the simple measurement of tidal volume, breathing frequency, or minute volume (the product of tidal volume and breathing frequency) provides limited information regarding the functionality of the lung because these values tend to be maintained near normal limits until respiratory disease is advanced.59
Measures of lung distensibility (dynamic compliance) and airway obstruction (pulmonary resistance) provide meaningful information regarding pulmonary health. One measures dynamic compliance (Cdyn) by dividing the tidal volume by the change in pleural pressure occurring between the start and end of inhalation. One measures pulmonary resistance by several different techniques, depending on whether one measures the resistance at peak airflow or at specific ventilatory volumes (e.g., 50% tidal volume), and calculates it by dividing airflow by the change in pleural (esophageal) pressure. Alterations in these two values can provide information on the nature of the lung disorder. For example, in obstructive disorders of the tracheobronchial tree, dynamic compliance decreases and pulmonary resistance increases. A decrease in dynamic compliance in the absence of a change in pulmonary resistance suggests that the lung parenchyma has been stiffened by alveolar disease or by obstruction of the peripheral bronchioles. (Recall that peripheral bronchioles, because of their immense cross-sectional area, contribute little to the resistance of breathing until the disorder is well advanced.) Conversely, an increase in pulmonary resistance in the absence of a change in dynamic compliance suggests that the obstruction exists in the upper airway, trachea, or bronchus.60
An alternative and less-invasive technique for measuring pulmonary function uses the forced-oscillation method (or its modification, the impulse-oscillation method). While the horse wears an airtight facemask, an external test signal is imposed on the horse’s breathing efforts that generate pressure-flow responses from the respiratory system. The mechanical properties derived from this technique over a wide range of frequencies (other than that of the horse’s own breathing frequency) include total respiratory system impedance (Zrs), respiratory system resistance (Rrs) and respiratory system reactance (Xrs).61,62 The impedance represents the impediment that the system presents to oscillatory flow and consists of Rrs (resistive properties of the respiratory system) and Xrs (elastic and inertive properties of the respiratory system).
Measures of airway hyperreactivity are often obtained in conjunction with pulmonary function testing.63–66 In this technique, one determines the dose of a nebulized bronchoconstrictor agent, such as histamine or methacholine, that causes a 35% increase in baseline Rrs or a 35% decrease in baseline lung compliance. As might be predicted, the dose to achieve this value in a horse with inflammatory airway disease or recurrent airway disease is much smaller than that needed to achieve a similar effect in a healthy horse. Measurement of airway hyperreactivity requires that the horse be sedated and outfitted with an airtight breathing mask. Laboratory assistants should wear protective masks to prevent inhalation of the bronchoconstrictor agent.
Pulmonary function testing of exercising horses and its use in assessing the poor performer is a new diagnostic approach offered at select referral centers. With the availability of high-speed treadmills and the ability to measure airflow during exercise,67 analysis of tidal volume, breathing frequency, dynamic compliance, lung resistance, end-expiratory lung volume, and flow-volume and pressure-volume loops may provide additional insights into the cause of the poor performance, the recognition of expiratory flow limitation, and the documentation of EIPH on respiratory mechanics.
Lung Biopsy
A histopathologic diagnosis may prove useful in the therapeutic management of certain lung disorders. Percutaneous lung biopsy has been used to investigate (1) disorders characterized radiographically by a pulmonary miliary pattern and (2) disorders for which radiographic or ultrasonographic results are compatible with pulmonary neoplasia or granuloma.68 Raphel and Gunson originally described the methods used,69 which has since been modified because of the routine use of ultrasound during tissue sampling. The horse is sedated and biopsy locations are selected after ultrasonographic confirmation of landmarks (heart, diaphragm) and assessment of tissue accessibility. The biopsy instrument is aseptically advanced through the intercostal space (cranial to the rib) to the depth of the desired tissues, and samples are obtained for culture and for fixation in 10% formalin. In a study comparing biopsy instruments (manual Tru-cut versus automated biopsy needle), the investigators found airway bleeding developed in approximately one third of the cases sampled with the manual device and one tenth of the cases biopsied using the automated instrument.70 The technique is not recommended in patients that are tachypneic, are in respiratory distress, exhibit uncontrollable coughing, or have bleeding disorders. The technique is not indicated in horses with pulmonary abscessation, pleuropneumonia, or pneumonia.68 In a recent review of percutaneous lung biopsies performed in 66 horses, this technique yielded a definitive antemortem histologic diagnosis of pulmonary disease in 82% of cases.71 In the same study, no complications were recognized in 91% of horses sampled.71 The most common complications observed with lung biopsy are coughing, epistaxis, pulmonary hemorrhage, tachypnea, and respiratory distress. Hemothorax or accidental sampling of abdominal organs (liver, stomach) may occur.70–72 When horses in respiratory distress are sampled using an automated instrument, epistaxis occurred in 13%, airway bleeding in 39%, and pneumothorax in 4% of patients.70
Thoracoscopy
Thoracoscopy or pleuroscopy is a diagnostic technique that is used in the standing sedated horse to visualize the intrathoracic structures that include the aorta, esophagus, intercostal vessels, sympathetic trunk, vagus nerves, lymph nodes, mainstem bronchi, pulmonary and azygous veins (right hemithorax), diaphragm, and dorsal and lateral surfaces of the lungs. The procedure is used to sample thoracic masses or nodules, to transect pleural adhesions, to place drains for the treatment of pleural abscesses, to repair diaphragmatic hernias, and to resect lung segments either for therapeutic or for diagnostic purposes.73–77 Using thoracoscopy, one is able to obtain larger biopsy sizes and hence assess parenchymal and peripheral airway morphologic features better.78
Thoracoscopy is performed using aseptic procedures. An area in the eighth to twelfth intercostal space just ventral to the serratus dorsalis muscle is surgically scrubbed and infiltrated with local anesthetic to place an endoscopic portal.79 Before insertion of the endoscopic portal (cannula), a pneumothorax is gradually created by inserting a teat cannula first through this intercostal space. The smaller cannula is replaced by a larger one through which a 10-mm (30-degree) rigid laparoscope connected to a video camera and light source can be advanced. Additional portals for biopsy or resection procedures are aseptically placed as needed. In most adult horses, the mediastinum is thought to be incomplete, thus a bilateral pneumothorax may ensue during this procedure. Hemodynamic effects of a unilateral thoracoscopy in healthy horses have been described.79 In sedated healthy horses, thoracoscopy (and pneumothorax) produces a mild systemic hypertension and arterial hypoxemia. After completion of the examination or retrieval of samples or both, the pneumothorax is reduced, the lung is reinflated, and the skin is closed after removal of the laparoscope. Analgesic and antimicrobial agents should be administered to the horse after this procedure.
DISORDERS OF THE UPPER RESPIRATORY TRACT
Sinus Disorders
SINUSITIS
Diagnosis and treatment of diseases of the paranasal sinuses are complicated by their large size, complex anatomy, difficult surgical access, and the advanced state of many diseases before a diagnosis is made.80
Anatomic Considerations
Six pairs of sinuses communicate with the nasal cavity: dorsal, middle, and ventral conchal and maxillary, frontal, and sphenopalatine.81 The frontal sinus has a large communication rostrally with the dorsal conchal sinus and thus forms the conchofrontal sinus. This drains through the frontomaxillary opening into the caudal maxillary sinus. The ventral conchal and rostral maxillary sinuses communicate over the infraorbital canal and drain through the nasomaxillary opening into the middle nasal meatus. The caudal maxillary sinus has a small opening medially into the middle conchal sinus. The sphenopalatine sinus drains over the infraorbital canal into the caudal maxillary sinus. In horses younger than 5 years, the last three cheek teeth—the first, second, and third molars (108-111 and 208-211)—occupy most of the maxillary sinus.80 As the horse ages and the residual root decreases, the sinus cavity enlarges, and its rostral limit approaches the infraorbital foramen.
The sphenopalatine sinus has thin walls and a close relationship to the brain, pituitary gland, and cranial nerves II, III, IV, V, and VI.82 The sinuses are lined by a respiratory epithelium—pseudostratified ciliated columnar—interspersed with goblet cells and underlying serous glands.83 The nasomaxillary opening is a narrow slit that is easily occluded from inflammation of the mucosa, and this occlusion can lead to accumulation of exudate within the sinuses. Most clinically important conditions involve the maxillary or frontal sinuses.
Causes
Primary sinusitis involves accumulation of exudate within the sinus cavities and is a sequela of viral or bacterial upper respiratory tract infections. Streptococcal (and rarely staphylococcal) organisms are the most frequent bacterial isolates. Secondary sinusitis is most commonly caused by dental disease or may be associated with sinus cyst, neoplasia, foreign body, or trauma.17,84–87
Dental sinusitis is associated with infection of the tooth root, fractured teeth, and patent infundibula.85 The first molar is the most commonly involved tooth.85,88,89 Sinus cysts act as a space-occupying lesion and have a variable wall that may be mineralized.87,90,91 Neoplasia of the paranasal sinuses include squamous cell carcinoma (which, in some cases, can arise in the oral cavity and spread to the maxillary sinuses), adenocarcinoma, bone and dental tumors, fibrosarcoma, and hemangiosarcoma.84,92 Although rare, fungal granulomata induced by Cryptococcus neoformans may cause secondary sinusitis.80
Clinical Signs
Clinical signs depend on the inciting cause, location, and chronicity of the disorder. Clinical signs include nasal discharge that may be unilateral or bilateral, malodorous, or mucopurulent; reduced nasal airflow; epiphora; and facial swelling.80,85 Sinus cysts and neoplasia are significantly more frequently associated with gross deformity of the nasal passages and facial bones than other disorders, which explains the high incidence of epiphora, reduced nasal airflow, and facial deformity in those conditions.85 Dental sinusitis is strongly associated with malodorous nasal discharge.80,85
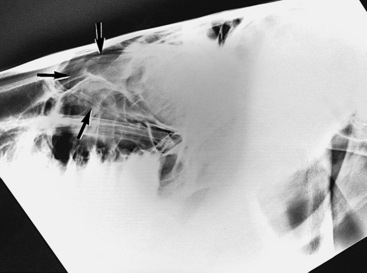
Diagnosis
Lateral and lateral-oblique radiographs (30 to 45 degrees to the horizontal plane) are helpful in diagnosing sinusitis and may demonstrate single or multiple horizontal fluid-air interfaces, abnormalities of the teeth, lysis of alveolar bone, or any combination of these signs (Figures 9-6 and 9-7).85,90 Dorsoventral radiographs are useful in selected cases (e.g., those with suspected empyema of the ventral conchal sinus). Strong diagnostic radiographic signs are present in approximately 57% of dental sinusitis cases.85 In contrast, radiographs are strongly diagnostic of sinus cysts in only 35% of cases.85 CT is the imaging diagnostic procedure of choice for diseases of the paranasal sinuses. The main advantage of CT is excellent cross-sectional imaging with no superimposition of structures.93,94 Nuclear scintigraphy is not significantly more useful than radiographs for the assessment of sinus disorders.58
Treatment
Treatment requires surgical removal of the primary cause (tooth, cyst, granulation tissue or neoplasia), the establishment of good drainage, and copious lavage. Nasofrontal surgical flaps provide good access to the majority of the paranasal sinuses95,96 and can be performed in the standing horse. In the largest published case series, resolution of signs was obtained after surgery in 84% of primary sinusitis, 78% of dental sinusitis, and 93% of sinus cysts.86 With neoplastic lesions, surgical resection or ablation achieves a low rate of success because of extensive infiltration of the neoplasm or recurrence of the tumor.97,98
PROGRESSIVE ETHMOIDAL HEMATOMA
Definition and Epidemiology
Progressive ethmoidal hematoma (PEH) is characterized by encapsulated, expansive masses that usually arise from the submucosa of the ethmoidal labyrinth.99,100 The mass is composed of blood and fibrous tissue, is encapsulated by respiratory epithelium,101 and may extend into the paranasal sinuses.99,101–103
The condition occurs in 4% to 6% of horses with diseases of the nose and sinus.85,100 The inciting factor in their development is not known, although they have been hypothesized to be associated with repeated episodes of submucosal hemorrhage. PEH appear bilaterally in 50% of cases and are more prevalent in older horses.103,104
Clinical Signs
The most common clinical sign caused by PEH is intermittent, serosanguineous discharge from the affected nasal passage.101,103,105 Other clinical signs include facial swelling, halitosis, respiratory distress, and coughing.101,103,105
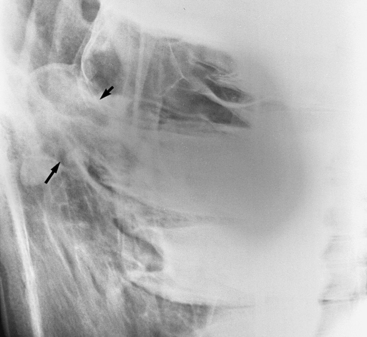
Diagnosis
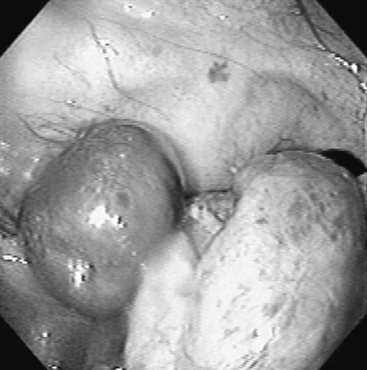
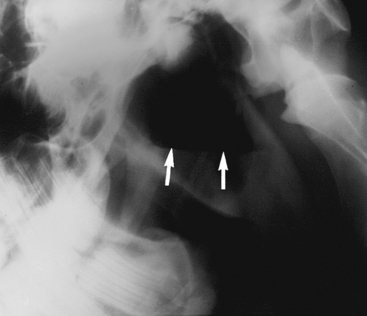
Diagnosis is based on the clinical signs, endoscopic examination, radiographic evaluation,101–103105 and, in some cases, CT findings to determine the extent of the lesion.106 Endoscopy (see Figure 9-2) reveals a variably green-colored, smooth glistening mass originating from the ethmoid region. The mass may protrude beyond the nasal septum (and in such cases may cause a bilateral nasal discharge). Radiographs reveal a space-occupying soft tissue density with smooth margins and may demonstrate extension into the paranasal sinuses (Figure 9-8). Confirmation of the diagnosis is by histopathologic study of the removed tissue,91,104 although the clinical appearance is highly suggestive.
Treatment
Treatment options include formalin injection, surgical resection, and laser ablation. Smaller lesions (<10 cm in diameter) can be treated effectively by the intralesional injection of 4% to 10% formalin.86,99,101,107 Most cases require multiple injections to achieve full resolution.86,101 This procedure can be performed in the standing horse, has a low complication rate, and is associated with 60% resolution after a median of five injections.101 Severe neurologic complications can, however, occur if the cribriform plate is fenestrated by the chronic pressure of a large PEH. CT may be indicated to determine the extent of the lesion before treatment.107
Surgical removal via a frontal sinus flap is associated with extensive hemorrhage and may require pre- or postoperative blood transfusions.99,100,105 A preoperative crossmatch is warranted, and the horse may also require a postoperative tracheotomy if extensive packing of the nasal cavity is necessary to effect hemostasis. After surgical removal, approximately 20% to 50% of ethmoidal hematomas recur.86,103–105 Postoperative complications may include facial incisional dehiscence, suture periostitis, facial bone sequestration, persistent nasal discharges, fungal sinus plaque formation, and encephalitis.103,105 Laser excision has also been used to treat PEH, with a recurrence rate of 20% described in one report.102 Recurrence is higher in bilateral PEH than other forms.
Guttural Pouch Disorders
ANATOMIC CONSIDERATIONS
The paired guttural pouches are diverticula of the external auditory tubes. Some investigators have suggested that the pouches play a role in cooling the arterial blood supply to the brain.108 Each pouch has a capacity of 475 ml and is divided into medial and lateral compartments by the stylohyoid bone.109 Each pouch fills on inspiration via the plica salpingopharyngeus. The mucosal lining of each pouch is secretory and covered by ciliated pseudostratified epithelium with goblet cells and glands.110 The medial compartment is three times bigger than the lateral compartment and contains the internal carotid artery and a fold that encloses cranial nerves IX, XI, and XII. The pharyngeal branch of the vagus nerve (X) traverses the floor of the medial pouch, and the cranial sympathetic ganglia are also found within it. The rectus capitus ventralis and longus capitus muscle run just medially to the guttural pouch, and tearing of these muscles after a poll injury can be identified endoscopically as blood in the associated pouch. The lateral compartment of the guttural pouch contains the external carotid and maxillary artery. Branches of the facial nerve (VII), vestibulocochlear (VIII), and mandibular branch of the trigeminal nerve (V) run close to the wall of the lateral compartment.
TYMPANY
Definition
Tympany is a nonpainful unilateral or bilateral distention of the guttural pouch by air that may produce an external swelling in the parotid region. Congenital tympany occurs in young foals (predominantly fillies), and acquired tympany uncommonly affects older horses.111
Causes
The cause of congenital guttural pouch tympany is unknown. Tympany is more likely a functional defect rather than an anatomic defect because no abnormality is visible endoscopically or during surgical exploration. The condition may have a genetic component in Arabians.112 Acquired tympany is typically associated with upper respiratory infection and is thought to be caused by swelling of tissues around the pharyngeal orifice, producing a unidirectional valve and trapping air or fluid within the pouch. This problem is transient, and rarely does pouch distention become severe.
Clinical Signs
Clinical signs depend on the degree of pouch distention and hence the degree of compression of the nasopharynx. If distention is significant, the foal may exhibit stertorous breathing, respiratory distress, dysphagia, nasal discharge, or evidence of pneumonia caused by aspiration. Regurgitation of milk from the nostrils also may be evident.113 Endoscopic examination may reveal significant compression of the nasopharyngeal area because of distention of the pouches.
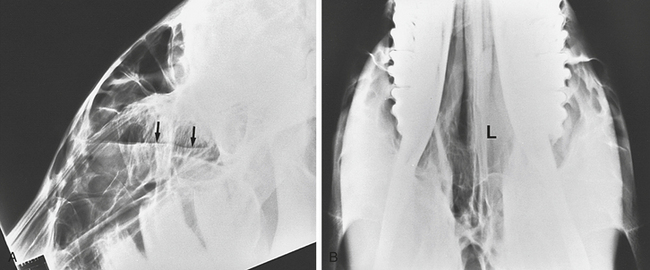
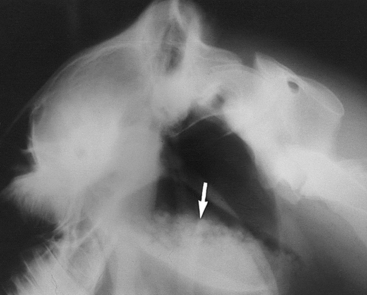
Diagnosis
Confirmation of the diagnosis is by the presence of tympanitic swelling in the area of Viborg’s triangle. Radiographs reveal a large, air-filled guttural pouch with or without fluid accumulation (Figure 9-9). The distinction as to whether the problem is unilateral or bilateral can be difficult to make. Catheterization of one guttural pouch should correct the problem if a unilateral tympany exists. Dorsoventral radiographic views may help in diagnosing bilateral involvement.
Treatment
Guttural pouch tympany can be relieved temporarily by percutaneous aspiration or placement of an indwelling catheter. Definitive treatment requires surgical correction. If percutaneous aspiration or indwelling catheter placement is unsuccessful, then surgical correction is required. For unilateral tympany, fenestration of the median septum, separating the two guttural pouches, by conventional or transendoscopic laser surgery allows movement of air into the normal pouch.114 For bilateral tympany, a fistula can be made with a transendoscopic laser, just dorsal and caudal to the guttural pouch ostium, over a Chambers catheter placed in the pouch. A Foley catheter is then placed for 7 to 10 days. This technique is associated with complete resolution of clinical signs with one (70%) or two (30%) surgeries.111 Prognosis for uncomplicated cases is good; however, the presence of aspiration pneumonia or dysphagia is associated with a guarded prognosis.
MYCOSIS
Clinical Signs
Many horses do not present until they experience significant spontaneous epistaxis caused by erosion of the fungal plaque through the arterial wall. Horses may also present with nasal discharge dysphagia, RLN, Horner’s syndrome, abnormal head extension, swelling in the parotid region, facial paralysis, mycotic encephalitis, and atlantooccipital joint infections.115–119 Although guttural pouch mycosis is uncommon in the young horse, it has been reported in foals younger than 6 months.120 Repeated episodes of epistaxis can be fatal in approximately 48% of horses, and definitive surgical treatment should be sought urgently.121
Pathogenesis
The events leading to the formation of fungal plaques are not known. Some researchers have suggested that aneurysmal dilations of the vasculature, visualized radiographically, provide a suitable environment for fungal organisms to proliferate.122 Fungal colonization leads to erosion of the underlying mucosa and vascular structures or injury to the adjacent nerves.
Diagnosis
Confirmation of the diagnosis is by endoscopic observation of a fungal plaque in the guttural pouch. Shortly after an acute bout of epistaxis, endoscopic examination can confirm the presence of blood at the guttural pouch ostium, but attempts to visualize the interior of the pouch may be unsuccessful if blood obscures the visual field. Some risk exists of dislodging a blood clot during endoscopic examination of the pouch.123 The presence of RLN or dysphagia should be documented at the initial examination because both are associated with a poor prognosis.124 RLN is usually permanent, although some horses recover from dysphagia over 6 to 18 months. Horses may also recover from facial nerve paralysis and Horner’s syndrome. Radiographs of the guttural pouch may show evidence of fluid accumulation or osteolytic changes in the stylohyoid bone or may suggest mycotic plaque formation.
Treatment
Treatment depends on surgical occlusion of the affected vessel or vessels. In an emergency situation with acute epistaxis, ligation of the common carotid artery is contraindicated if the hemorrhage is from the internal carotid artery; flow in the internal carotid artery, from the circle of Willis, will be increased with ligation of the common carotid artery. Several different techniques have been advocated for occlusion.124–129 Current therapies of choice use a coil or intravascular balloon to obliterate arterial flow proximal and distal to the fungal lesion.117 The placement of transarterial coils, via an incision in the common carotid artery under fluoroscopic guidance, is associated with 84% survival and 71% return to performance.124 If fluoroscopic guidance is not available, correct placement of the occluding balloon is essential to prevent postoperative complications, including blindness.130,131 Correct placement can be difficult in the presence of aberrant vessels.127,132 The fluoroscopic technique allows identification of such vessels before the occluding coils are placed.
EMPYEMA
Definition
Empyema is an accumulation of exudate within the guttural pouches and is usually a sequela of upper respiratory tract infections (Streptococcus spp.). Streptococcus equi subsp. equi was isolated from 32% of cases evaluated for empyema in a large case series.133 Empyema may also result from the rupture of abscessed retropharyngeal lymph nodes into the pouches or may accompany cases of guttural pouch tympany.113 The condition may be unilateral or bilateral.
Clinical Signs
The most common presenting sign is a nasal discharge that may be unilateral or bilateral.133 Other clinical signs include lymphadenopathy, painful distention in the parotid region, stertorous breathing, dysphagia, and occasionally epistaxis.113 Inspissation of the material may occur with chronic infections, forming masses called chondroids in approximately 20% of cases.133
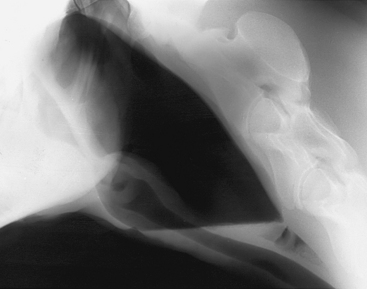
Diagnosis
Confirmation of the diagnosis is by radiographic examination or endoscopy. Radiographs demonstrate a fluid line or opacity in the pouch (Figure 9-10). Inspissated material also may be evident radiographically (Figure 9-11). Endoscopic examination may reveal a purulent material at the pharyngeal orifice of the auditory tubes and within the medial or lateral compartments of the guttural pouches. Distortion of the pharynx may occur if distention is significant.
Treatment
Given that S. equi subsp. equi may be involved, the clinician should isolate affected horses and take precautions to prevent spread of the bacteria (see discussion on strangles). Treatment may entail medical and surgical modalities. In acute cases, daily irrigation with physiologic saline is usually effective.133 The horse should be sedated to enhance lowering of its head and to improve drainage of flush materials. Administration of both topical and systemic benzylpenicillin improves treatment success. A gelatin-penicillin mixture can be instilled through a catheter inserted endoscopically into the guttural pouch.134 A 50-ml mixture is prepared by first heat dissolving 2 grams of gelatin in 40 ml of sterile water and then cooling to 45° to 50° C. To this mixture is added 10 million units of sodium benzylpenicillin that have been reconstituted with 10 ml of sterile water. The mixture is dispensed in syringes and stored overnight at 4° C to set. For each treatment, 25 ml of the mixture is instilled.
One may attempt removal of chondroids with an endoscopic snare, preventing the complications or risk of surgery and minimizing the cost of treatment. However, this task is difficult if the chondroids are numerous.134,135
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree