CHAPTER 18 Disorders of the Reproductive Tract
SEASONAL REPRODUCTIVE CYCLE
Anestrus
Anestrus usually occurs during the shortest days of winter and is characterized by reproductive incompetence in most mares. Measurements of hypothalamic gonadotropin-releasing hormone (Gn-RH)1–4 indicate a low hypothalamic Gn-RH content and secretion rate during anestrus. Pituitary gonadotropin secretion consequently is reduced, with low or undetectable circulating concentrations of luteinizing hormone (LH) and follicle-stimulating hormone (FSH).5–7 Although pituitary stores of FSH appear to be adequate throughout anestrus,8 this is not the case with LH. The messenger ribonucleic acid (mRNA) encoding LH subunit production becomes undetectable in pituitary tissue during anestrus.9,10 Thus pituitary gonadotropins (FSH and LH) are low during anestrus because of suppression of the releasing signal from the hypothalamus (Gn-RH), plus inactivation of the gene encoding LH synthesis in the pituitary. The result is, not surprisingly, loss of function of the ovaries. Ovarian activity, including follicular development and hormone production, is minimal, with few follicles greater than 5 mm in diameter and undetectable circulating concentrations of estradiol and progesterone.
As might be expected from this reduction in circulating hormones, sexual receptivity in mares during anestrus tends to be reduced or lacking altogether. Given the lack of circulating hormones, the passivity of mares in response to teasing not surprisingly is highest in winter.6
Vernal (Springtime) Transition
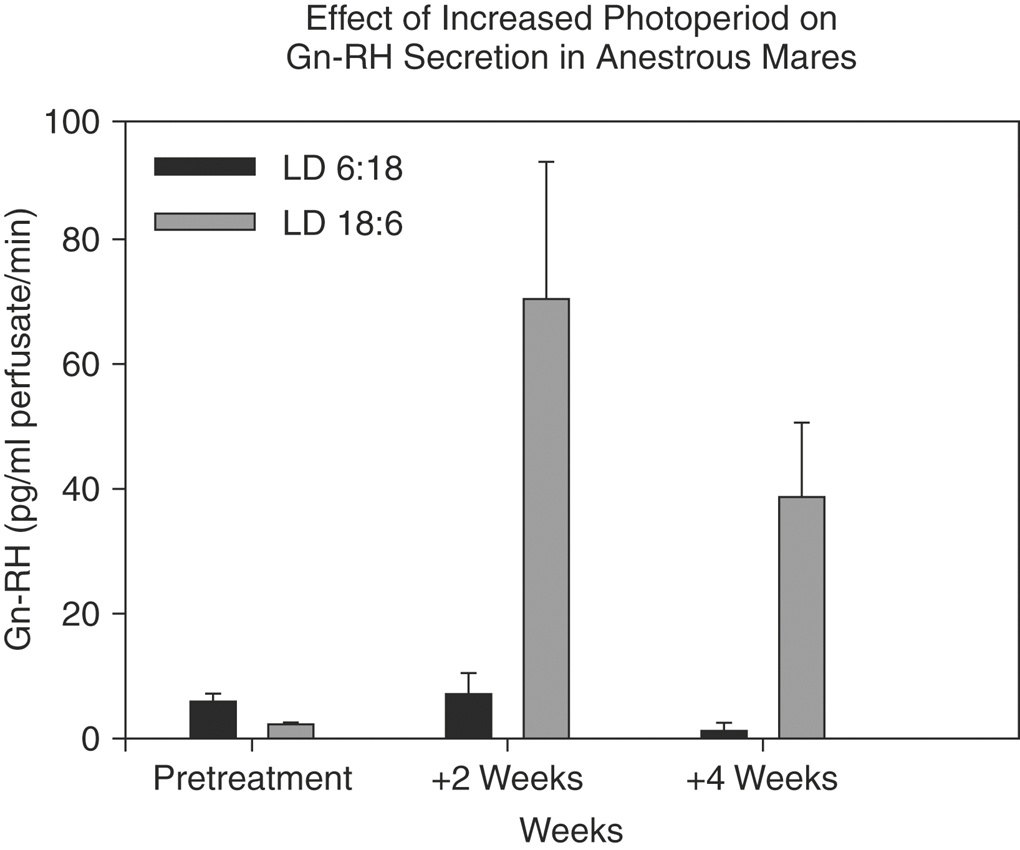
The vernal transition of the annual reproductive cycle, which begins sometime around the first of the calendar year, is the most troublesome commercially. The first event in transition—likely the initiating one—is an increase in hypothalamic Gn-RH observable within 1 week after the winter solstice2 or 2 weeks after exposure to an artificially increased photoperiod several weeks before the solstice (Figure 18-1).11 Whether the increase in hypothalamic Gn-RH represents a response to increasing day length, refractoriness to short day length, or some other factor remains unresolved.
Hart, Squires, Imel, et al.8 reported that the increased Gn-RH secretion shortly after the winter solstice leads to increased FSH secretion from available pituitary stores but that LH levels remain low. Sherman, Wolfe, Farmerie et al.9 explained this phenomenon, stating that the mRNA encoding LH subunit synthesis was undetectable in the winter and early spring. The third major event during the vernal transition, after hypothalamic Gn-RH secretion and subsequent release of pituitary FSH stores, is follicular development. During vernal transition the size and number of manually detectable follicles increases.12,13 The follicular population changes from only two or three small follicles (5 to 10 mm in diameter) to six or more midsize follicles (15 to 25 mm), with the diameter of the largest follicle exceeding 35 to 40 mm.12 A major problem early in the breeding season is that many of these large vernal transition follicles do not ovulate. In ponies an average of 3.5 ± 0.7 large (<30 mm) anovulatory follicles develop in sequence before the first ovulation of the year.6 These anovulatory vernal transition follicles are the cause of high reproductive inefficiency during the springtime, and it is impossible to discern, by palpation or ultrasound, whether they will be ovulatory. Consequently, many mares are sent to the stallion inappropriately, which adds to to the frustration and expense of breeding. Furthermore, mares with chronic failure of uterine clearance likely suffer most from such an inappropriate breeding, and the penalty to the breeder is often greater expense aimed at resolving uterine contamination.
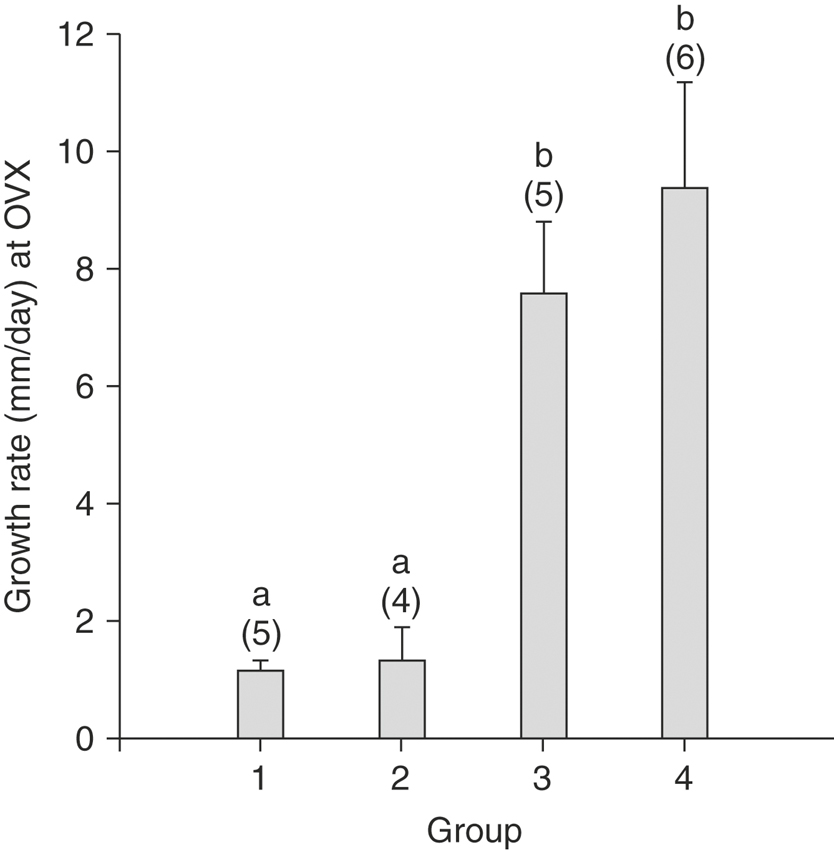
The first several vernal transition follicles are different from fully functional follicles during the breeding season. Although they attain a diameter comparable with that of preovulatory follicles of the breeding season (>30 mm), they do so much more slowly. Careful tracking of follicles reveals that the first and second large follicle of the year grow at a rate of only 1 to 2 mm per day (Figure 18-2), compared with growth rates of about 10 mm per day for follicles that go on to ovulate.14 Therefore one may assess the potential functional status of springtime follicles by frequently monitoring their growth.
The slower growth rate of vernal transition follicles reflects other metabolic deficiencies, including poor vascularity compared with that of ovulatory follicles and significantly smaller granulosa cell content.15 The poor granulosa cell investment of early vernal transition follicles is significant in that the granulosa cell layer contributes substantially to the steroidogenic capabilities of the follicle. Thus steroid production by the early vernal transition follicles is limited. The follicles do not produce estradiol, as monitored by peripheral blood, follicular fluid, or in vitro production rate. Estradiol concentration is low or undetectable in peripheral plasma until 5 or 6 days before the first ovulation of the year.7,14,16 One can measure circulating estradiol to discriminate between nonfunctional and functional (ovulatory) follicles.
Renewed LH synthesis and secretion, also manifested by a large surge 5 to 6 days before the first ovulation of the year, is associated with the rise in estradiol. Sharp, Grubaugh, Davis et al,17 and Cleaver and Sharp18 have demonstrated that administration of estrogen advances this secretion of LH in mares during early vernal transition, and Sharp, Wolfe, Cleaver et al.10 further demonstrated that estrogen administration to ovariectomized mares at the same time of year stimulated the mRNA encoding for the LH α and β subunits. In other words, estrogen administration was associated with activation of the LH gene. However, as Freedman, Garcia, and Ginther5 and Peltier, Robinson, and Sharp7 have demonstrated, the increase in peripheral LH occurs at approximately the same time in mares with and without ovaries. Thus the role of estrogen in bringing about the resurgence of LH synthesis and secretion remains unclear. Furthermore, one must resist the temptation to use estrogen to hasten LH synthesis in mares during the early vernal transition because of the negative effects of estrogen on FSH secretion. The clinician may initiate LH synthesis only to find that it inhibits follicular development.
Autumnal Transition
The transition from reproductive competence in the breeding season to reproductive quiescence characteristic of anestrus is perhaps the least understood part of the annual seasonal reproductive cycle in mares. The period of reproductive decline has a much greater variance during the fall than does the period of reproductive renewal in the spring. The variance is understandable from the evolutionary aspect, because mares likely faced little pressure to reproduce in the fall because their offspring would face more difficult survival at foaling the next year. Although this is an oversimplification, the autumnal transition may be characterized simply as the gradual loss of gonadotropic support for ovarian function, resulting in reduced follicular development. The final act of the autumnal transition may be development of a large preovulatory follicle that fails to ovulate.19
MORPHOLOGIC BEHAVIORAL AND HORMONAL CHANGES OF THE ESTROUS CYCLE
Estrus
BEHAVIOR
Understanding estrous behavior, as monitored by teasing with a stallion, is an important foundation to any breeding program. The word estrus, coined by Walter Heape,20 is thought to come from the Greek word Oestridae, a genus of the gadfly, because locomotor activity of many female ungulates increases during estrus. Estrous behavior is associated temporally with increasing estrogen concentrations peripherally but also may be associated with the concomitant decline in progesterone levels. Support for the idea that estrogen may not be essential for estrous behavior comes from observations of estrous display in mares with inactive ovaries and from ovariectomized mares during anestrus.21 The displays of estrous mares reflect a wide range of behaviors and extents, however, and successful breeding farms often owe their success in part to careful monitoring and record keeping for each mare. Among the signs observed during estrus are elevation of the tail in the presence of the stallion, eversion of the vulvar lips to expose the clitoris (“winking”), squatting with rotation of the pelvis, urination, and remaining calm (i.e., not moving away) in the immediate presence of a stallion. Recording behavioral signs tends to take the decision making out of the watching process and potentially provides a more precise record of progress through the estrous cycle.
OVARIAN MORPHOLOGY
Morphologic changes during estrus can be used to monitor the progress toward ovulation. The equine ovary is structurally different from that of other species in that the cortical elements containing the primordial follicles are located centrally in the ovary instead of peripherally, as in all other species that have been studied so far. This central position of potential ovulatory follicles presents a dilemma in that ovulation cannot occur at the site of follicle development. Most of the surface of the equine ovary is covered with mesothelium, which apparently does not allow the tissue remodeling required for rupture of the follicle. Instead, ovulation in the mare always occurs at a specific site, called the “ovulation fossa. which, ” is located ventrally on the ovary. The bean shape of the equine ovary creates a recessed fossa area in which germinal epithelium is located. This inside-out structure of the mare’s ovary likely contributes to some of the unusual aspects of the equine estrous cycle, including the period of prolonged estrus, the large preovulatory follicle size, and the prolonged increase in LH that precedes ovulation. A logical assumption is that the structure of the ovary led to co-evolution of a hypothalamic-pituitary axis that was able to present gonadotropins over a prolonged duration. The ovarian structure requires extensive tissue remodeling for a developing follicle to reach the restricted area of the fossa where it can ovulate. The extended gonadotropin secretion seems likely to be an important part of the tissue remodeling process that precedes rupture of the follicle. Administration of Gn-RH analog to stimulate endogenous LH release, or human chorionic gonadotropin (hCG; LH activity), results in increased levels of tissue remodeling enzymes in the follicle within 24 hours.22 This prolonged process presents challenges to the breeder or veterinary practitioner who is attempting to schedule breeding at a time appropriate to the expected ovulation.
TUBULAR REPRODUCTIVE TRACT
Changes in intrauterine edema are evident, as monitored by ultrasound imaging, in the pattern of fluid (edema) present in the endometrial folds during estrus (Figure 18-3). The appearance of edema often is used to indicate estrus, but an important note is that the relationship between edema and ovulation is inverse. Edema is maximal several days before ovulation and then begins to dissipate. Ovulation occurs 3 to 4 days after peak edema scores are exhibited and the edema pattern is in the process of dissipation.23 Because estrogen stimulates edema rapidly (within 6 hours), the presence of edema appears to be a useful indicator of estrogen secretion. However, dissipation of the edema is an active process, requiring the presence of ovaries, and likely involves progesterone as well. Far more intriguing, then, is the question of the mechanism of dissipation of edema. What is the purpose of edema in the first place if an active process exists for its dissolution before ovulation?
HORMONES
Estrus in mares is characterized by increasing estrogen and a prolonged elevation in LH. The prolonged elevation of LH,24 often reaching peak concentrations after ovulation, may reflect the need for extensive tissue remodeling of the ovaries for ovulation to occur. Estradiol reaches peak concentrations of greater than 30 to 40 pg/ml 2 to 3 days before ovulation. Evidence exists for a positive feedback effect of estradiol on LH,25 but evidence also suggests that LH rises spontaneously after corpus luteum (CL) regression and the loss of progesterone negative feedback. This raises the question of whether estrogen positive feedback is necessary for the ovulatory increase in LH or whether it merely serves to enhance the default situation of elevated LH in the absence of progesterone.
POSTPARTUM PERIOD
Immediately after expulsion of the fetus, the equine uterus begins the process of involution, and this continues for several days after passage of the placenta. During this time detection of fluid within the uterine lumen by ultrasound is not abnormal. However, the uterine fluid (lochia) and debris should dissipate 7 to 10 days after parturition in preparation for “foal heat” in the mare.26 One should not detect fluid by ultrasound 10 to 14 days postpartum, and the uterus should be restored to nearly prepregnancy condition within 3 to 4 weeks.
Foal heat is defined as the first period of sexual receptivity after parturition and usually begins 7 to 9 days after foaling.27 The first ovulation after foaling occurs on average at 9 days after foaling. Depending on the efficiency of uterine involution, the fertility of this foal heat varies. If significant fluid is present within the uterus at foal heat, breeding the mare is not recommended. In addition, studies regarding the effect of season on first postpartum ovulation suggest that mares that foal in January and February tend to have longer intervals from foaling to the first fertile ovulation than mares that foal in late spring.28 After the foal heat most mares have a 30-day heat that occurs approximately 21 days after the foal heat and may represent a more fertile estrous cycle.
In addition to the effects of season, a condition known as lactational anestrous may develop immediately after foaling. In some cases the condition may persist until weaning (4 to 6 months). Determining whether the lactational anestrous results from the foal nursing or the time of year (seasonal) is important. Limited studies have been performed in which foals were removed from mares at varying time points after parturition. Research indicates that removal of the foal hastens the onset of estrus. Foal removal has been associated with elevated LH concentrations and increased ovarian follicle diameter.29
SIGNALMENT AND HISTORY
History and signalment may suggest possible problems and guide the diagnostic approach (Table 18-1). The use of drugs during an athletic career can affect the horse’s breeding future. The use of anabolic steroids can change behavior and external genitalia. There might also be a downregulating effect on the hypothalamic-pituitary-gonadal axis that has to reverse over time. The use of an anti-GnRH vaccine (Equity; Pfizer Animal Health, West Ryde, Australia) is an effective way to inhibit estrus behavior in fillies. Unfortunately a number of fillies have had prolonged ovarian inactivity into their breeding career.1 The author had one cytogenetically normal mare that was retired from breeding with four seasons of ovarian inactivity after the use of the vaccine.
TABLE 18-1 Details of Information to be Gathered During History Taking
| History | Comments |
|---|---|
| General health | Vaccination, deworming, foot care, dental care, nutritional history, systemic diseases, and management |
| Athletic | Level of activity, age at which athletic career ended, any known drug usage |
| Cycling | Length of estrus, expression of estrus, interovulatory interval |
| Breeding | Number of cycles bred, breeds per cycle, natural or artificial insemination, fertility of stallion, age at which the mare was last bred |
| Pregnancies | Number of diagnosed pregnancies and outcomes |
| Foaling | Any history of dystocia, type of management |
| Prior reproductive examinations | Diagnoses, treatment, management |
| Prior reproductive surgeries | Nature of surgery, date performed and outcome |
| Drug use during breeding season | Ovulation induction, estrous cycle manipulation |
BEHAVIOR
The season of the year should be considered when assessing behavior in the mare.2 Assessing the mare’s response to the teaser while in estrus is important for future breeding use. Shy or aggressive behavior toward the teaser by the mare while in physiological estrus might limit natural breeding options.
EXAMINATION OF EXTERNAL GENITALIA
Vulval integrity is important in the formation of an effective seal against contamination of the genital tract with air, urine, feces, and potential pathogens.3 Vulval conformation is assessed by digital palpation adjacent to the vulval lips to locate the caudal bony pelvic brim. Pascoe’s description of a Caslick index has been modified to produce a simple assessment technique for vulval conformation:4
TRANSRECTAL PALPATION AND ULTRASOUND EXAMINATION OF THE GENITAL TRACT
Palpation of the genital tract requires a methodical approach. The author usually locates the uterus and palpates structures in the following sequence: right uterine horn, right ovary, right uterine horn, left uterine horn, left ovary, left uterine horn, uterine body, cervix. Cervical size and consistency are assessed by pressing the cervix down against the pelvic floor. Cervical relaxation can most effectively be measured on a sliding scale, from 0% relaxed (i.e., tightly closed, tubular cervix) to 100% relaxed (i.e., indistinguishable from adjacent uterus).5
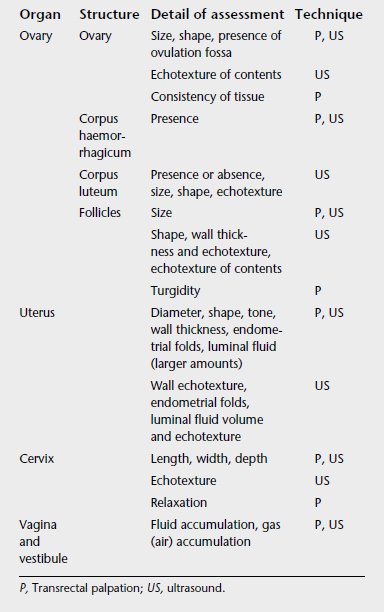
Ultrasound examination should be considered a routine part of a reproductive tract examination. Ultrasound examination allows the use of electronic calipers for measuring structures and allows assessment of tissue and fluid characteristics. Table 18-2 presents comparative information on the application of palpation and ultrasound for assessing various genital tract structures.
TABLE 18-2 Application of Transrectal Palpation and Ultrasound of the Genital Tract for Assessing Various Parameters
DIAGNOSIS OF OVARIAN PATHOLOGY
Abnormal ovarian enlargement should be suspected if an ovary is greater than 10 cm in diameter and the increased size cannot be attributed to recent ovulation or a follicle and persists for more than a month.5,6 Large ovaries may be associated with physiologic causes (e.g., persistent follicles, hematoma, multiple CLs) or pathologic processes (e.g., neoplasia, abscess).
Neoplasia more commonly affects one ovary, although it can affect both, and typically results in a variable degree of alteration in ovarian size, shape, and consistency. There appears to be no consistent pattern in the change affecting a neoplastic ovary, with the exception of teratomas in which the diagnosis may be based on detection of germ tissues, including cartilage, hair, and bone. Other neoplasias may present as single large cystic or multicystic structures or even solid tissue. In many cases the diagnosis of ovarian neoplasia is based on persistent unilateral ovarian enlargement with obliteration of the ovulation fossa and an inactive or normally functioning contralateral ovary.7,8 There might be a history of aberrant ovarian activity before the development of the tumor.9 A granulosa cell tumor may produce varying levels of testosterone, estrogen, and inhibin9,10 but rarely produce progesterone. The measurement of α-inhibin might be more accurate for diagnosis than measuring αβA-inhibin.11
Functional ovarian tumors such as granulosa-theca cell tumor (GTCT) may manifest as masculinization of appearance and behavior and increased plasma inhibin and testosterone concentrations.12 The ultrasonographic appearance of the GTCT is characteristic, varying from uniformly dense to cystic.13,14
Anovulatory follicles often grow to an unusually large size and fill with blood, organizing into a structure with the ultrasonographic appearance of a hematoma that gradually recedes over time. Some of these structures appear to develop a thick rim of tissue resembling luteal tissue, with the mare entering a diestrus-like state. Others do not develop a thick rim of tissue, and the structure may appear to be inert. There is a lack of consensus regarding terminology, with some authors distinguishing anovulatory hemorrhagic follicles (no luteinization) from luteinized unruptured follicles (development of luteal rim). Others suggest that the two structures are variations of the same condition.15 The condition appears to be more common in mares approaching winter anestrous and in pregnant mares under the influence of equine chorionic gonadotropin. Anovulatory follicles occur in 8% of all estrous cycles16 but in 5% of estrous cycles in the early ovulatory season and 20% of estrous cycles in the late ovulatory season.17 The ultrasonographic appearances of ovulatory and anovulatory follicles are largely the same.
Failure of the normal process of luteolysis may result in prolonged luteal maintenance. Diagnosis depends on confirmation of nonpregnant status and the presence of a CL for a period longer than the normal diestrus length. This condition, termed pseudopregnancy, must be differentiated from cases in which a mare is ovulating between examinations and exhibiting an apparently persistent CL. Luteolysis depends on prostaglandin (PG) release from the uterus,18–20 which in turn might depend on a uterine clock being set for PG release. An ovulation during diestrus does not always set this clock, and the resultant CL can last for more than 2 months.19,20 Other reasons for failure of luteolysis include release of PG when the CL is still immature and unable to respond to PG (before 6 days of age), failure of PG to be released at the proper time, and insignificant release of PG from the endometrium in mares with degenerative endometritis.21
Very small and inactive ovaries may be secondary to seasonal anestrus, reproductive senescence in old mares, pituitary gland dysfunction, severe malnutrition, administration of anabolic steroids, administration of anti-GnRH vaccine, or congenital infertility resulting from sex chromosome abnormalities.1,22 Sex chromosome abnormalities are often associated with hypoplasia or segmental aplasia of the tubular tract. Karyotyping may be used to further investigate such mares. Chromosomal abnormalities have also been reported from subfertile mares that have foaled previously and have no detectable abnormalities of the genital tract. In light of this fact, karyotyping may be considered in mares in which routine diagnostic procedures fail to explain subfertility.
Individuals with 63,X karotype tend to be small in stature, phenotypically female, with small uterus and ovaries, and no sexual behavior.23–26 However, some might show irregular estrus behavior, and the uterus palpably and histologically can be within normal limits.24,27 Plasma estrogen levels are low and plasma LH levels are normal to high.24 Sex reversal is a disorder that occurs in horses and can have a variety of abnormalities.28 The two most common forms of sex reversal are mare phenotype with male karyotype 64,XY and male phenotype with female karyotype 64,XX. There can be a wide phenotype variation with both conditions.29,30
X trisomy is relatively rare in the horse. The mare is infertile; shows estrus behavior and normal external genitalia; and is phenotypically female, with small, thin walled uterus and small hypoplastic ovaries.23,25,31,32
Mosaics and chimeras occur when the individual has more than one cell line with different chromosomal makeups. 63,X/64,XX mosaics are phenotypically female; the uterus varies in development, but ovaries may be small and nonfunctional. These horses may show estrus behaviour, an endometrial biopsy reveals glandular hypoplasia, and the clitoris may be enlarged.23–25,26,33
DIAGNOSIS OF UTERINE PATHOLOGY
Endometritis
Ultrasonography is very useful for evaluating presence, quantity, and echotexture of uterine luminal fluid. Fluid echotexture is correlated with the amount of debris or white blood cell infiltration into the fluid and is more common in older mares.34 Table 18-3 presents a grading system for recording uterine fluid echotexture.35 The presence of grade I, II, or III fluid during any stage of the cycle indicates endometritis. The presence of fluid in diestrus results in a lower pregnancy rate and a higher embryo loss rate,36 presumably caused by high levels of PGF2a in the fluid from the neutrophils.37 The presence of more than 2 cm of fluid in the lumen during estrus, the presence of fluid during diestrus, and fluid 72 hours after insemination are strong indicators of mare susceptibility to endometritis.38 Table 18-4 presents a grading system for recording the volume of intrauterine fluid.
TABLE 18-3 Classification System for Recording Uterine Fluid Echotexture
| Grade | Ultrasound Appearance | Gross Appearance |
|---|---|---|
| I | White (hyperechoic) | Thick and creamy |
| II | Light gray | Milky |
| III | Black with white specks | Obvious sediment in fluid |
| IV | Black (anechoic) | Clear fluid |
TABLE 18-4 Grading System for Recording Volume of Intrauterine Fluid
| Classification | Maximum Fluid Depth (mm) | Gross Description |
|---|---|---|
| VS (very small) | 1 to 2 | Barely detectable |
| S (small) | 1 to 5 | Often focal |
| M (moderate) | 5 to 20 | Obvious fluid |
| L (large) | >20 | Immediately apparent |
Complete absence of detectable uterine luminal fluid during estrus is associated with absence of cytologic inflammation in 99% of cases.39 The significance of clear (grade IV) luminal fluid is less clear. Small or very small volumes of clear fluid during estrus are likely to be normal, particularly if present during early estrus before complete cervical relaxation and maximal uterine drainage. Large volumes of clear fluid during estrus, fluid that persists into late estrus, or smaller volumes during early diestrus suggest an increased predisposition to endometritis and reduced pregnancy rates compared with mares without fluid.39–41 Luminal fluid visible more than 12 to 20 hours after breeding indicates mating-induced endometritis.22,42 This condition has recently been reported in 10% to 15% of mares being bred on a commercial Thoroughbred stud farm.43
Endometrial Cysts
Circumscribed, discrete collections of clear fluid within the uterine lumen indicate endometrial cysts or a conceptus. Cysts may occur on the endometrial surface or within the endometrium. Cysts may be single, discrete structures closely resembling a conceptus or complex, compartmentalized structures with irregular borders. Cysts that protrude into or occlude the uterine lumen may reduce fertility by interfering with embryo mobility and maternal recognition of pregnancy.36,44,45 Cysts within the endometrium may interfere with gland function.
Properties of an early conceptus that can be used to distinguish it from a cyst include the following: movement between days 10 through 16 post ovulation; spherical, symmetric appearance; rate of growth (cysts are assumed to grow little if at all); and appearance of the embryo proper after about day 20. In addition, there are palpable changes in the tract that are consistent with early pregnancy (e.g., increased uterine tone and closed, elongated cervix) that would not be expected to occur in a nonpregnant mare with endometrial or lymphatic cysts. Any association between cysts and reduced fertility remains unclear.46 Large cysts or large numbers of cysts may interfere with embryonic movement, maternal recognition of pregnancy, and early placentation. Extensive glandular cystic change may also adversely affect uterine gland function and compromise establishment or normal development of pregnancy.
Pyometra
Pyometra is a chronic condition with mucoid or mucopurulent material within the uterine lumen, with or without cervical occlusion. The condition is not dependent on the presence of a CL, as it usually is in other species, and does not typically result in any systemic signs of illness. Pyometra may be mistaken for pregnancy on palpation as a result of fluid distention of the uterus but can be diagnosed effectively with ultrasound.47
Adhesions
Adhesions and scarring involving the endometrium may result from infusion of irritating solutions into the uterus and perhaps as a result of trauma associated with dystocia and its correction. Luminal adhesions involving the cervix have similar initiating causes. The role of chronic endometritis or iatrogenic causes such as mechanical curettage in uterine or cervical adhesions is less clear. Adhesions may reduce fertility by interfering with function of the uterotubal junction or with embryo movement and maternal recognition of pregnancy.45
Focal uterine lesions may respond well to surgical removal using a biopsy instrument or laser surgical equipment guided by hysteroscopy.48 If the lesions are extensive or affecting the deeper layers of the endometrium, then the prognosis may be poor and treatment not warranted. Cervical adhesions are commonly managed by manual disruption and then repeated application of topical ointment containing antibiotic and steroid in an attempt to prevent recurrence. In many horses, cervical adhesions tend to recur once treatment stops.
Ventral Uterine Sacculation
Ventral sacculation is associated with a loss of normal muscular contractile activity in the uterine wall, with the uterine wall forming sacculations ventrally at the base of one or both uterine horns. The condition is more common in older, multiparous mares and may indicate increased risk of endometritis caused by loss of the normal contractile mechanisms responsible for expelling uterine luminal fluid. This may also be linked to mares that have a more ventral oriented uterus. When the uterocervical angle is more ventral than horizontal, the mare is more prone to fluid accumulation because of a reduced ability to clear fluid.49
Vaginal Speculum Examination
Vaginoscopy is useful for detection of vaginal hyperemia, suppurative exudates, persistent hymen, urine pooling, varicose veins, vaginal trauma, rectovaginal defects, and cervical defects. Although examination with a vaginal speculum may be performed at any stage of the cycle, inspection of mares suspected of pooling urine is best performed during estrus because estrogenic relaxation of the genital tract and perineal region is maximal at this time.50 Vaginal speculum examination is an important component of the BSE but should not be considered a routine procedure to be performed repeatedly in normal mares.
Manual Examination of the Vagina and Cervix
Examination of the genital tract is incomplete without manual exploration of the vagina and cervix. The major benefit in this procedure is detection of cervical defects. Once the hand is inserted into the anterior vagina, the forefinger is placed into the cervical lumen and the thumb in the vaginal fornix. The hand is then rotated and the entire circumference of the cervix palpated between finger and thumb to feel for disruption in the cervix resulting from lacerations or damage to submucosal layers that might interfere with the ability of the cervix to close effectively. Cervical disruption is significant only if it prevents the cervix from forming an effective seal during diestrus and pregnancy. A partial tear involving the external os of the cervix may not require treatment if the cervix can still maintain an effective seal. For this reason, assessment of the severity of cervical disruption should be performed when the mare is in diestrus.51 Alternatively, the diestrus tone can be duplicated by the administration of progesterone or progestagen for a few days to close the cervix.
Endometrial Culture and Cytology
A variety of instruments and techniques have been described for culturing the genital tract. The lack of consistency in methodology has almost certainly contributed to the lack of consensus regarding the role and interpretation of genital tract cultures. Interestingly, in a group of mares infected with Klebsiella, the normal uterine swab was less sensitive at detecting infection (27/60) than use of a tampon (55/60).52 A gynecologic brush was superior to a cotton swab or aspiration in the detection of endometritis. The cotton swab often produced a false-negative result.53 In the author’s experience, aspiration or analysis of a small volume saline lavage can sometimes yield more information than a cotton swab. Sterile digital sampling of the endometrium has been described as a quick stallside test for cytology.54 Care should be taken not to collect neutrophils from caudal areas of the reproductive tract and interpret that as endometritis.
Cultures may be taken from multiple sites in the genital tract, including endometrium, cervix, vagina, clitoral fossa, and clitoral sinuses. The isolation of bacteria from the anterior vagina is associated with reduced fertility.55 Genital tract culture performed to investigate possible endometritis or as part of a BSE should be taken from the endometrial lumen and not from the vagina or cervix. Endometrial culture should be performed before any other invasive procedure in a BSE to reduce the risk of inadvertent contamination of the uterine lumen before taking the culture sample.
A guarded culture rod should be used to minimize the risk of contamination with material from sites other than the uterine lumen.56 Single- or double-guarded culture rods may be used. It is also possible to take an endometrial culture from a uterine biopsy sample as long as the culture sample is taken in a sterile manner and before placing the tissue into fixative. There is a lack of consensus on the optimal time of the cycle for obtaining endometrial culture and cytology samples. Some experts recommend performing endometrial cultures on the first or second day of estrus, when uterine secretions are increasing and the flushing action of the uterus is just beginning.57,58
Mares with endometritis have been reported to accumulate free luminal fluid in late diestrus, and this represents an alternative time for endometrial culture.36 The practitioner should carry the guarded culture rod into the vagina and through the cervix beside one finger. The rod is then passed cranial to the finger, into the endometrial lumen, and the culture tip pushed through the guard and rolled against the endometrial surface for 30 to 60 seconds. The culture tip is withdrawn into the rod before withdrawing the culture rod to prevent contamination during removal from the genital tract.
Interpretation of endometrial culture results is difficult. False-positive and false-negative results are common. Several recommendations can be made to minimize the likelihood of an erroneous interpretation. Culture results should be interpreted in conjunction with results of concurrent endometrial cytologic examination. In addition, the practitioner should consider whether the organisms recovered represent pure or mixed growth and whether growth can be considered heavy or light. Acute endometritis is more commonly associated with mixed bacterial growth on culture than pure growth.59
In the absence of cytologic evidence, it is particularly difficult to interpret culture results. Recovery of relatively pure or heavy cultures of the following organisms may be considered indicative of endometritis: β-hemolytic streptococci, hemolytic Esherichia coli, Pseudomonas spp., Klebsiella spp., and Candida spp.60,61 Recovery of other organisms without concurrent cytologic information should be viewed with more suspicion.
Endometrial cytology is used by some practitioners as a rapid screening test for detection of endometrial inflammation in mares at the beginning of the breeding season or before breeding. When it was first described in the mare,62 the detection of neutrophils was correlated with positive bacteriologic findings. When no or low numbers of neutrophils were detected, bacteriologic findings were more often negative.62 Mares with cytologic evidence of inflammation had lower 28-day pregnancy rates than mares with normal cytologic results, irrespective of culture results. Day 28 pregnancy rates were also lower in mares that had bacteria isolated from their uterus even if cytologic results were normal.63
Numerous techniques have been described for collecting cytologic samples. The approach used by the author is simple, rapid, and feasible in busy clinical practice. A guarded culture rod with a snap-on cap (Kalayjian: Kalayjian Industries Inc., Long Beach, California) is used to collect a culture sample. The swab tip is withdrawn back into the sheath and the rod rolled against the endometrium to collect fluid and cells in the cap. After the rod is withdrawn from the mare, the cap can be cut off and tapped against a slide to make the smear from the small drop of fluid.64 The smear is dried and stained using any commercial staining kit (e.g., Diff-Quik; (American Scientific Products, McGaw Park, Ill.). If no endometrial cells are seen on an initial inspection of the slide, the sample may not have been collected from the uterus and another sample should be taken. The presence of polymorphonucleocytes (PMNs) is generally believed to indicate bacterial endometritis, but the most appropriate methodology for quantifying PMNs remains subject to debate. Suggestions for criteria to diagnose endometrial inflammation include observation of more than 1 or 2 PMNs in five high-power microscope fields (400×) or more than 1 PMN per 10 endometrial cells in more than one area of the slide.65,66 In most mares with endometritis, there are large numbers of PMNs evident on cytologic evaluation.
Endometrial Biopsy
Preparation for endometrial biopsy is the same as for culture or cytology. Diestral samples are generally preferred over estral samples because physiologic changes in the endometrium during estrus make the slides more difficult to interpret.67,68 However, samples may be taken at any stage of the cycle as long as the information on the stage of cycle is provided and the person reading the slide has experience in assessing equine biopsy slides. In the absence of clinically detectable pathology involving the uterus, a single endometrial biopsy sample is representative of the entire endometrium.69 However, another study found that even in clinically normal mares endometrial biopsy scores varied with the site selected.70
A simpler vaginal technique is useful for collecting biopsy samples when there is no need to sample from a specific site.67,68,71 The instrument is introduced into the uterine lumen, and the hand is left in the vagina with the index finger in the cervical lumen. The tip of the instrument is advanced about 2 to 3 cm into the uterine lumen with the jaws closed. The jaws are opened and the instrument advanced an additional 1 to 2 cm. The instrument can then be deviated slightly to one side and the jaws closed to collect a sample. The sample is taken from the cranial uterine body close to the bifurcation. This approach has the advantage of being quick and simple. It allows the operator to return for a second sample immediately if the first sample is too small, whereas the rectovaginal technique often results in gross contamination of the instrument and vulva during the procedure, making a second sample impossible unless the mare is cleaned again and a second sterile instrument is available.
The specimen is removed from the biopsy basket using a small-gauge needle and transferred to fixative solution, preferably Bouin’s fixative or 10% buffered formalin. Samples placed into Bouin’s fixative should be transferred to 10% formalin or 80% ethanol after 12 to 24 hours for optimal retention of cell detail and tissue integrity.64
Endometrial fibrosis is a permanent degenerative change occurring around glands (fibrotic nests) or gland branches and also adjacent to the basement membrane of the luminal epithelium. Fibrosis often forms in concentric layers around gland branches or glands, and the number of layers of fibrosis is related to the severity of the degenerative change.72 Fibrosis may be localized or diffuse, and the more widespread or severe the change, the more adverse the effect on gland function and fertility. These degenerative changes appear to be closely associated with age rather than parity.34,73–75 Severe degenerative endometrial fibrosis can occur in the older maiden mare that has not had the challenges of pregnancy and exposure to semen.74,75
Cystic gland distention may be seen in normal mares during anestrus. When diagnosed in mares during the breeding season, it is considered a pathologic change and is associated with reduced fertility. Glandular fibrosis may also be associated with cystic distention of the affected glands. Extensive fibrosis interferes with uterine gland function and may result in early embryonic death.76
Endometrial atrophy is associated with an extremely poor breeding prognosis.67,68 Large empty spaces on the biopsy slide must be interpreted with caution, because they may be artifacts associated with sample processing. If the spaces appear to be lined with an endothelial cell layer, this indicates lymphatic stasis. Widespread lymphatic stasis may be associated with reduced contractile capability within the uterus, a doughy feel to the uterus on transrectal palpation, and reduced fertility.64
Assessment of severity and distribution of pathologic changes allow the sample to be classified into one of four diagnostic and prognostic levels based on a modified version of the original three-level system proposed by Kenney.72,77
Use of paired biopsy samples taken at the initial diagnostic workup and again 4 weeks after completion of treatment appears to improve the usefulness of the biopsy as a prognostic indicator of a mare’s fertility.74,75 Mares that were classified as grade III pretreatment and that improved to grade II after treatment achieved a foaling rate of 40%, whereas mares that were still grade III after treatment had a 0% foaling rate. This is likely to be a result of improvement in reversible pathologic changes.74,75 This approach allows effective use of the grade III categorization on the follow-up biopsy to justify recommending that such mares be culled from the breeding program.
Uterine Endoscopic Examination: Hysteroscopy
Hysteroscopy allows direct visual inspection of the uterine lumen through a flexible fiberoptic endoscope. Hysteroscopy is indicated when other diagnostic procedures do not detect a cause for subfertility or to further examine a mare with a suspected uterine luminal lesion.78,79
Excessive distention of the uterus may cause discomfort and an elevated heart rate and should be avoided.80 Mares subjected to hysteroscopy appear to be at risk of developing subsequent endometritis.80 Care should be taken to sterilize the equipment before use; the author suggests that mares be examined during the subsequent estrus period to check for evidence of endometritis.
HORMONAL ASSAYS
Progesterone is produced by ovarian luteal tissue. Serum progesterone concentrations are low during estrus and begin to rise 12 to 24 hours after ovulation, peaking between days 5 to 10 after ovulation.81,82 Progesterone assays in nonpregnant mares can be used to assist in determining the stage of the cycle and to confirm that ovulation has occurred. They are also used as an indirect method of pregnancy diagnosis, but false-positive results are common.
Progesterone is necessary for pregnancy maintenance, and primary luteal insufficiency has been linked to pregnancy loss.40,83,84 Low levels of progesterone are associated with increased pregnancy loss, and higher levels of progesterone are associated with successful pregnancies in mares that habitually aborted twins.85 Older mares appear to require a higher level of progesterone to maintain pregnancy.59 Progesterone levels on day 7 were significantly lower in mares with periovulatory intrauterine fluid accumulation and significantly lower in mares that underwent embryonic loss.40 The presence of luminal fluid in diestrus was associated with a lower progesterone level and increased embryonic loss, indicating endometritis as a cause for lower progesterone levels36
Some researchers recommend that progesterone concentration in peripheral blood of pregnant mares be above 2.5 to 4 ng/ml for normal pregnancy maintenance.86 Single samples of peripheral blood concentrations are difficult to interpret because repeated sampling of normal pregnant mares has shown that blood levels vary widely over short periods of time.81,82
Thyroid gland dysfunction has been linked to subfertility in mares with recommendations to measure total serum thyroxine (T4) or thyroxine response to administration of thyroid-stimulating hormone (TSH). Thyroxine is significantly affected by season and reproductive status.87,88 Thyroxine is seasonally regulated when mares are kept on a constant energy balance out of season.89 Although thyroidectomy of pony mares did not have any adverse effect on reproductive performance,90 mares that continued to cycle out of season had higher levels of thyroxine than anestrous mares.88,91 Mares with low thyroxine levels were more likely to enter anestrus after parturition.88
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree