12 Disorders of the Neurologic System
Neurologic Examination
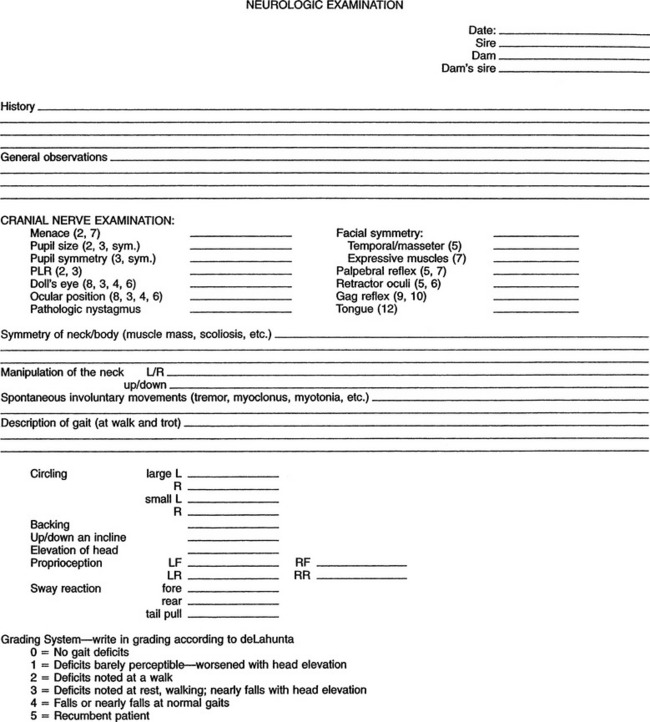
The neurologic examination should be considered part of the physical examination. The author prefers to begin the examination at the head and proceed caudally to the tail. The examiner should proceed in a consistent fashion and should record findings in an orderly manner to avoid any part of the examination being omitted. Figure 12-1 shows a sample format. One may use the craniocaudal approach for all animals, whether ambulatory or recumbent.
The equine neurologic examination procedure has been described in detail elsewhere.1–13 The author follows the format developed by Mayhew,1 which divides the examination into five categories: (1) the head and mental status, (2) gait and posture, (3) neck and forelimbs, (4) trunk and hindlimbs, and (5) tail and anus. The functional divisions of the nervous system include the sensory, integration, and motor systems.
examination
Postural abnormalities of the head and neck sometimes can be difficult to distinguish from head tilt. Horses with torticollis of the head and neck may have a congenital abnormality of the vertebrae or may have injured the muscles of the neck region. Damage to the dorsal gray columns can result in abnormal head and neck posture (acquired scoliosis) that has been described as a result of parasitic migration such as Parelaphostrongylus tenuis.4 Careful examination, including palpation, should help identify fractures of the cervical vertebrae or painful muscles caused by trauma or an injection reaction.  Neck pain may manifest as a reluctance to move the head and neck through a full range of motion. In the absence of pain or mechanical limitations to movement, normal horses should be able to turn their head to retrieve feed offered at the lateral thorax on each side, elevated to a level above the withers, or lowered to a point between the front legs just beneath the pectoral muscles. In some horses, radiographs of the cervical vertebrae may be useful to confirm a fracture or osteomyelitis. Blindness sometimes can lead to an abnormal head or neck posture.
Neck pain may manifest as a reluctance to move the head and neck through a full range of motion. In the absence of pain or mechanical limitations to movement, normal horses should be able to turn their head to retrieve feed offered at the lateral thorax on each side, elevated to a level above the withers, or lowered to a point between the front legs just beneath the pectoral muscles. In some horses, radiographs of the cervical vertebrae may be useful to confirm a fracture or osteomyelitis. Blindness sometimes can lead to an abnormal head or neck posture.
The cerebellum helps regulate rate and range of motion. With damage to this area a horse often shows fine resting tremors of the head that worsen with intentional movements.3 One may normally observe this tremorous movement of the head and neck in the newborn or young foal, but it is not normal in older foals and adult horses. In young Arabians and a few other breeds, a condition of cerebellar abiotrophy has been reported.1–3 Horses with this condition show a hypermetric gait, failure to blink when exposed to bright light, and absence of a menace response.
 Examination of the eyes should include evaluation of the blink or menace response, the ability of the horse to negotiate in a strange environment, pupillary light response, and in some cases a funduscopic examination. If a horse has a lesion of the eye or optic nerve, then the lesion will result in complete or partial ipsilateral blindness. To develop contralateral blindness, the horse would have to have a lesion in the optic tract or the lateral geniculate nucleus.1
Examination of the eyes should include evaluation of the blink or menace response, the ability of the horse to negotiate in a strange environment, pupillary light response, and in some cases a funduscopic examination. If a horse has a lesion of the eye or optic nerve, then the lesion will result in complete or partial ipsilateral blindness. To develop contralateral blindness, the horse would have to have a lesion in the optic tract or the lateral geniculate nucleus.1
Examination of the face should include evaluation of pupil size and symmetry, which are under control of the autonomic nervous system and are affected by environmental light and level of fear or excitement. To test the pupillary response, one should direct a light in each eye and note the constriction of the pupil. Identifying a consensual response in the horse is often difficult when one works alone. A swinging light test has been described.1 In the performance of this test, the light is shone alternately into each eye and the more powerful direct pupillary light response is observed. A unilateral lesion affecting the afferent tracts in the eye, optic nerve, optic chiasm, or optic tract to the level of the midbrain will result in ipsilateral dilation when the light reaches the affected eye. Moving the light from side to side takes advantage of the ipsilateral light response being stronger than the consensual response. The examiner can perform this procedure alone. These reflexes are in the brainstem and are not affected by lesions in the visual cortex.
in each eye and note the constriction of the pupil. Identifying a consensual response in the horse is often difficult when one works alone. A swinging light test has been described.1 In the performance of this test, the light is shone alternately into each eye and the more powerful direct pupillary light response is observed. A unilateral lesion affecting the afferent tracts in the eye, optic nerve, optic chiasm, or optic tract to the level of the midbrain will result in ipsilateral dilation when the light reaches the affected eye. Moving the light from side to side takes advantage of the ipsilateral light response being stronger than the consensual response. The examiner can perform this procedure alone. These reflexes are in the brainstem and are not affected by lesions in the visual cortex.
One possible cause of asymmetrical pupils in a horse is Horner’s syndrome. Horner’s syndrome is the collection of clinical signs associated with dysfunction of the sympathetic nerve supply to the head and neck. The sympathetic nerve supply to the head includes upper motor neurons in the caudal hypothalamus, parts of the midbrain and pons, as well as parts of the medulla oblongata. Axons from these centers descend in the cervical spinal cord to the preganglionic neurons in the cranial thoracic spinal cord. From here, preganglionic axons leave the cord and join the paravertebral sympathetic trunk, where they ascend up the neck to synapse in the cranial cervical ganglion in the guttural pouch.4 This syndrome includes ptosis of the upper eyelid, miosis of the pupil, and protrusion of the third eyelid (nictitating membrane). In addition, the horse has unilateral facial sweating, increased facial temperature, and hyperemia of the nasal and conjunctival membranes. These signs should alert the examiner to the possibility of a previous perivascular injection, a guttural pouch infection, or damage to the sympathetic nerves in the vagosympathetic trunk, which courses from the cranial thoracic spinal cord through the thoracic inlet and upward to the orbit.5 Loss of sympathetic innervation to the head results in the triad of clinical signs described, with the most prominent initial sign being increased sweating, which is not what one would expect and the exact cause of which is not well understood.6,7
Focal sweating in a horse indicates involvement of the peripheral pre- or postganglionic sympathetic neurons. Identification of this problem also can aid in the anatomic localization of a neurologic lesion.1
After careful examination of the mental status and behavior of the horse and of the head, neck, and cranial nerves, the examiner should look for asymmetry of the muscles of the trunk, pelvic region, tail, and anus. Identification of focal sweating, focal muscle atrophy, or increased or decreased pain responses is helpful in localizing signs. In addition, the horse has two cervical reflexes. The cervicofacial reflexes result in a local twitch and drawing back of the lips (smile reflex) when one pricks the skin along the side of the neck down to the region of the second cervical vertebra. Below the region of the second cervical vertebra one should observe a local response.14
one pricks the skin along the side of the neck down to the region of the second cervical vertebra. Below the region of the second cervical vertebra one should observe a local response.14
Evaluation of Gait
Evaluation of the gait of the horse should include examination of postural reactions and may include evaluation of spinal reflexes in young or small horses. Although they may appear weak and ataxic, foals are ambulatory within hours after birth, making it possible to evaluate gait. Because postural reactions are sometimes difficult to interpret in horses, using gait abnormalities to help localize a lesion is essential. The author nearly always places the feet and limbs in an unusual position to observe the response of the horse. Gait abnormalities that are observed commonly in horses with neurologic disease include ataxia, spasticity, and weakness or paresis.1–3,4,15,16
The examiner should observe the movements of each limb carefully to determine if a deficit is present and should assign a grade to the deficit. The author uses a system modified from the grades described by deLahunta3 and Mayhew.1 The severity is graded between 0 and 5. Grade 0 means no gait deficits. A grade 1 deficit requires careful observation to be certain the gait abnormality is caused by a neurologic dysfunction. Grade 2 deficits are mild to moderate but obvious to most observers as soon as the horse begins to move. Grade 3 deficits are obvious and are exaggerated during the negotiation of a slope or with head elevation. Grade 4 gait deficits may cause a horse to fall or nearly fall. When attempting to walk, an animal with these severe deficits often displays abnormal positioning while standing in its stall. Grade 5 horses are recumbent.
One must realize that many horses that have minimal (grade 1 or mild grade 2) deficits often can race or perform other athletic activities.13 The examining veterinarian has the responsibility of separating a neurologic from a musculoskeletal gait deficit and helping the owner determine the usefulness of the horse. For example, a horse with gait deficits up to grade 3 may be useful as a broodmare or breeding stallion (if the horse is handled by careful, knowledgeable persons who understand the risks and have the facilities to accommodate a horse in need of special management). Stallions with this degree of impairment may require assistance when mounting and dismounting mares. If the breed association allows artificial insemination, then the stallion may be easier to manage.
ce:small-caps>Localization of the Lesion
Cervical spinal cord disease includes gait and proprioceptive deficits in all four limbs with no signs of brain, brainstem, or cranial nerve deficits. One should note that horses with cervical vertebral stenotic myelopathy might have mild pelvic limb deficits with minimal or barely detectable signs in the thoracic limbs.16
Horses that have peripheral nerve injury (Table 12-1), EMND, or polyneuritis equi show evidence of weakness, muscle atrophy, and in some cases selected areas of sensory loss. Primary muscle diseases such as exertional rhabdomyolysis, myotonia (congenita or dystrophica), and hyperkalemic periodic paralysis are covered elsewhere in this book (see Chapter 11). These conditions sometimes can mimic a neurologic problem.
Description of Normal Gaits
A walk is a natural four-beat gait in the horse. At this gait the normal horse has three feet on the ground at all times. Therefore the walk is a stable gait. Overtracking and interference may occur if the conformation of the horse is abnormal (e.g., with long limbs and a short body) and not as a result of neurologic disease.11 The trot is a two-beat symmetrical gait in which the diagonal limbs are in contact with the ground at the same time. If one examines the horse on hard pavement, then the trot is the most helpful gait to distinguish lameness from a neurologic gait deficit. The pace is a two-beat symmetrical gait in which the legs on the same side of the body strike the ground simultaneously, resulting in significant truncal sway. In horses that have neurologic disease, identifying ataxia with truncal sway, which is often accompanied by pacing, is common. In horses with subtle ataxia, one observes pacing when horses walk with the head held in an extended position. Whenever one observes a horse pacing, the pacing may indicate an underlying neurologic disorder.
 Stringhalt often begins as an abrupt onset of excessive flexion of one or both rear limbs. In some horses the condition may worsen and result in frequent episodes of the foot hitting the abdomen. The condition has been reported for a long time and in some areas of the world may occur as an outbreak.17 The clinical syndrome is similar to the movement of a horse with a tibial neurectomy with unopposed flexion of the hock and extension of the digit. In the case of Australian stringhalt, the forelimbs and neck muscles may be involved. Stringhalt deserves mention in this chapter because when the examiner observes this gait, the examiner should be certain no other signs of neurologic gait deficits exist. The primary condition usually can be corrected by a tenectomy of the lateral digital extensor tendon, including a portion of the muscle belly. The underlying cause of the disease may be a sensory neuropathy, a myopathy, or primary spinal cord disease. The defect likely affects the neuromuscular spindle, as well as the efferent and afferent pathways controlling muscle tone.10
Stringhalt often begins as an abrupt onset of excessive flexion of one or both rear limbs. In some horses the condition may worsen and result in frequent episodes of the foot hitting the abdomen. The condition has been reported for a long time and in some areas of the world may occur as an outbreak.17 The clinical syndrome is similar to the movement of a horse with a tibial neurectomy with unopposed flexion of the hock and extension of the digit. In the case of Australian stringhalt, the forelimbs and neck muscles may be involved. Stringhalt deserves mention in this chapter because when the examiner observes this gait, the examiner should be certain no other signs of neurologic gait deficits exist. The primary condition usually can be corrected by a tenectomy of the lateral digital extensor tendon, including a portion of the muscle belly. The underlying cause of the disease may be a sensory neuropathy, a myopathy, or primary spinal cord disease. The defect likely affects the neuromuscular spindle, as well as the efferent and afferent pathways controlling muscle tone.10
 The evaluation of the major peripheral nerves in the horse is also an important part of the neurologic examination (Table 12-1). The important points to remember are that damage to a peripheral nerve can result in sensory and motor deficits in the area supplied by the nerve and that focal muscle atrophy follows within a short time after damage to one of these nerves. The examiner is referred to other portions of this book for a more detailed anatomic description of these nerves in the horse; however, a dropped elbow joint with radial nerve paralysis, inability to fix the stifle with femoral nerve paralysis, and atrophy of the supraspinatus and infraspinatus muscles (sweeney) with damage to the suprascapular nerve are classic examples of what to expect with peripheral nerve injuries.1–3
The evaluation of the major peripheral nerves in the horse is also an important part of the neurologic examination (Table 12-1). The important points to remember are that damage to a peripheral nerve can result in sensory and motor deficits in the area supplied by the nerve and that focal muscle atrophy follows within a short time after damage to one of these nerves. The examiner is referred to other portions of this book for a more detailed anatomic description of these nerves in the horse; however, a dropped elbow joint with radial nerve paralysis, inability to fix the stifle with femoral nerve paralysis, and atrophy of the supraspinatus and infraspinatus muscles (sweeney) with damage to the suprascapular nerve are classic examples of what to expect with peripheral nerve injuries.1–3
Oformation, Flow, and Function of Cerebrospinal Fluid
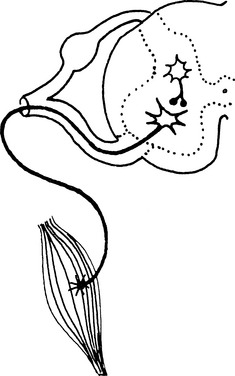
CSF is an actively transported ultrafiltrate of plasma that bathes the CNS.1 The CSF is located in the ventricles of the brain and subarachnoid space of the spinal canal (Figure 12-2) and originates from the choroid plexus and ependymal lining of the ventricles.2 CSF flows from the ventricular system up and over the cerebral hemispheres and through the subarachnoid space surrounding the spinal cord.1 Pulsation of blood in the choroid plexuses forces the CSF in a caudal direction. The rate of CSF production varies between 0.017 ml/min in cats to 0.5 ml/min in human beings3 and is independent of the blood hydrostatic pressure. The rate of CSF production for horses has not been determined. Osmotic and hypertonic solutions such as mannitol and dimethyl sulfoxide (DMSO), when added to blood, decrease CSF production and decrease CSF pressure and edema.1
Collections of arachnoid villi (arachnoid granulations) are located in the venous sinus or the cerebral vein and absorb CSF. CSF absorption is related directly to the pressure gradient between the CSF and venous sinus. When CSF pressure exceeds venous pressure, these villi act as one-way ball valves, forcing CSF flow to the venous sinus.4
CSF functions to suspend the brain and spinal cord for protection, regulate intracranial pressure, and maintain the proper ionic and acid-base balance.1
collection of Cerebrospinal Fluid
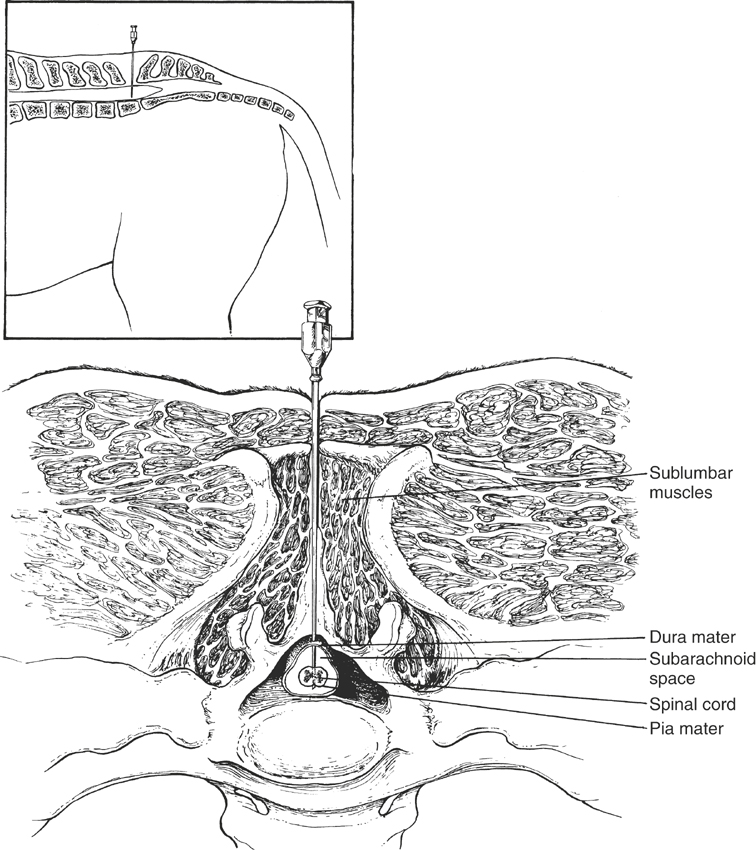
Techniques for collecting CSF in horses have been described in detail elsewhere.1–5 One may collect CSF from the lumbosacral site in a standing horse sedated with an intravenous injection of 0.2 to 0.5 mg/kg xylazine or 0.01 to 0.02 mg/kg butorphanol (or a combination of both drugs). Alternatively, one can collect CSF from the atlantooccipital site of an anesthetized horse. In foals and recumbent adult horses, one can collect CSF while the animal is restrained and heavily sedated with 1.0 mg/kg xylazine and 0.2 mg/kg butorphanol, both administered intravenously. If the lesion is localized to an area above the foramen magnum (at least cranial to the second cervical vertebra), then CSF collected from the atlantooccipital site will be more diagnostic. If the lesion is localized to an area below the foramen magnum (caudal to the second cervical vertebra), then CSF collected from the lumbosacral site will be more diagnostic. These differences result from the craniocaudal circulation of CSF. Collecting CSF from both sites at the same time and comparing the findings may be helpful in cases in which neuroanatomic localization of the lesion is difficult.5
examination of Cerebrospinal Fluid
Reference values for CSF in horses have been reported,6–11 but each laboratory should determine its own reference ranges. CSF determinations that are helpful in evaluating horses with neurologic diseases include pressure, appearance, cellular content, protein concentration (total protein, albumin, immunoglobulin G [IgG]), enzyme activity (creatine kinase [CK], aspartate aminotransferase [AST], lactate dehydrogenase), and lactic acid concentration.
Pressure
One can measure opening CSF pressure before withdrawal of CSF by attaching a manometer tube with a three-way stopcock to a properly placed spinal needle, allowing the CSF to rise within it.12 Because the cranial and vertebral cavities are enclosed in a rigid bony compartment, changes in blood pressure or volume can cause a concomitant increase in CSF pressure. Thus increased CSF pressure may occur from venous compression or jugular occlusion. Venous compression causes increased blood volume in the cranial cavity and compression of the CSF space, leading to increased CSF pressure. One can use jugular occlusion clinically to increase CSF pressure and facilitate collection of CSF fluid.13 The jugular compression maneuver, or Queckenstedt’s phenomenon, can help one diagnose compressive lesions, neoplastic lesions, or an abscess along the spinal cord. With compression and obliteration of the subarachnoid space by a compressive lesion in the cervical or thoracic spinal cord, jugular vein compression does not cause a CSF pressure increase in the lumbosacral site.1
Increased CSF pressure may occur after injury, after systemic changes in blood pressure, and in the presence of an intracranial space-occupying mass such as a tumor, abscess, hemorrhage, or edema. One must provide an adequate airway after injury and during surgery to prevent hypoxia-mediated cytotoxic cerebral edema and vasogenic cerebral edema.14 Cytotoxic edema is caused by inadequate cerebral oxygenation and leads to neuronal, glial, and endothelial cell swelling. Such reactions are especially important during long surgical procedures or recumbency in which respiratory hypoxia and poor alveolar ventilation (hypercapnia) may occur. Hypercapnia increases cerebral blood flow in the cranial cavity and CSF pressure and may worsen existing cerebral edema.1
Increased CSF volume may occur in hydrocephalus, which is defined as an increased volume of CSF and can be classified as compensatory or obstructive.15 Compensatory hydrocephalus is an accumulation of CSF in areas in which brain tissue has been destroyed and may occur from brain injury or inflammation. Hydranencephaly is destruction of brain tissue from a viral or other infectious agent and results in severe accumulation of CSF.15 CSF pressure in compensatory hydrocephalus usually does not increase.1
Obstructive hydrocephalus is an accumulation of CSF in the ventricles from an obstruction to CSF outflow or absorption. Cerebral aqueduct malformation may lead to obstruction of ventricular CSF outflow. Inflammatory lesions, especially of the arachnoid villi, result in decreased absorption of CSF and increased CSF pressure. The white matter is affected more severely than the gray matter in obstructive hydrocephalus, but the cerebral cortex usually is spared.1 CSF pressure in obstructive hydrocephalus usually increases. The presence of an abnormally high opening pressure that drops by 25% to 50% after removing 1 to 2 ml of fluid suggests a space-occupying intracranial mass or spinal cord compression cranial to the site of collection. The removal of more fluid would risk causing tentorial herniation.12
Appearance
One can evaluate the appearance of CSF immediately after collection. Normal CSF is clear and colorless and does not clot, and newsprint is visible through it.16 CSF may be red tinged from blood contamination after a traumatic tap or from preexisting trauma to the CNS. In the case of a traumatic tap, the CSF usually will clear if allowed to flow for several seconds (about 0.5 to 1.0 ml). With preexisting trauma and secondary hemorrhage, the supernatant of CSF after centrifugation is xanthochromic.
Other causes of CSF xanthochromia include increased protein concentration (150 mg/dl)16 and direct bilirubin leakage from serum in horses with high serum bilirubin concentration. In addition, indirect bilirubin may leak across a damaged blood-brain barrier. Clots in the CSF are abnormal and may be caused by increased amounts of fibrinogen resulting from inflammation.1
Turbid CSF may indicate an increased number of white blood cells (>200/μl), an increased number of red blood cells (RBCs) (>400/μl), epidural fat, bacteria, fungal elements, or amebic organisms.1 Cytologic evaluation and cultures can help differentiate causes of turbidity.
Cytologic Evaluation
One can use a standard hemocytometer to obtain a complete blood count (CBC). In addition, a sedimentation chamber requiring 0.5 to 1.0 ml of CSF is a rapid method for cytologic evaluation.17 One must perform cell counts and cytologic evaluation within 30 minutes to avoid degeneration. If cell counts or cytologic evaluation cannot be performed immediately, then one can mix a portion of the sample with an equal volume of 50% ethanol to preserve cellular characteristics.12 CSF from normal horses and foals usually contains fewer than 10 white blood cells per microliter. However, much variation occurs in CSF white blood cell counts in horses.9,10
Most studies show no differences in white blood cell counts in normal CSF samples obtained from the atlantooccipital space as compared with samples obtained from the lumbosacral space.10 However, one study did show a slightly higher total white blood cell count in CSF obtained from the lumbosacral site, but white blood cell counts in all of those horses were less than 10 per microliter.6
Small mononuclear cells (70% to 90%) and large mononuclear cells (10% to 30%) predominate in equine CSF. Rarely, one may see neutrophils in horse CSF. Increased CSF large mononuclear phagocytes are visible in horse CSF in diseases of axonal degeneration.9 One may see increased CSF neutrophil numbers in encephalomyelitis, bacterial meningitis, parasitism, and diseases with extensive inflammation. Occasionally, in severe inflammatory diseases of the neurologic system or parasitism, eosinophils may be visible.9,17,18 In some cases, cytologic evaluation of CSF may reveal specific agents causing neurologic disease such as fungal organisms,16 bacteria, or tumor cells. Although CSF cytologic examination may support a diagnosis of neurologic disease, it may not yield a specific causative diagnosis.
Protein Concentration and Composition
Normal total protein values range between 20 to 124 mg/dl, depending on the measuring method used1,6–10 (Table 12-2). Total protein concentration is higher in lumbosacral CSF compared with atlantooccipital CSF.6 A difference of 25 mg/dl of protein between the atlantooccipital and lumbosacral spaces may suggest a lesion closer to the space with greater spinal fluid protein.10 Proteins in the CSF are derived from the peripheral blood and include albumin, IgG, and possibly other globulins. Increased CSF albumin and IgG concentrations may occur with damage to the blood-brain barrier or increased intrathecal production of IgG. One can determine CSF albumin and IgG concentrations by electrophoresis and radial immunodiffusion, respectively, and can compare these with serum concentrations.6 Special low-level radial immunodiffusion plates (VMRD Inc, Pullman, WA) are available to quantify CSF IgG concentration. One can calculate the albumin quotient (AQ) ([Albc]/[Albs] × 100) and IgG index ([IgGc]/[IgGs] × [Albs]/[Albc]) to determine blood-brain barrier permeability and intrathecal IgG production.6 Increased intrathecal IgG production (increased IgG index) may occur in inflammatory spinal cord disease such as equine protozoal myeloencephalitis (EPM), bacterial meningitis, some tumors, and EMND. Increased blood-brain barrier permeability (increased AQ) may occur in equine herpesvirus-1 (EHV-1) after necrotizing vasculitis.7 Determining blood-brain barrier integrity is also important in planning therapy. If the blood-brain barrier is damaged, then pharmacologic agents such as penicillin that do not normally penetrate the blood-brain barrier will penetrate a disrupted blood-brain barrier and attain bactericidal CSF concentration.
TABLE 12-1 Localization of peripheral nerve injuries in horses
| Nerve | Clinical Signs | Common Causes of Injury |
|---|---|---|
| Suprascapular | Injury is termed sweeney. Eventual atrophy of supraspinatus and infraspinatus muscles. Lateral subluxation (popping) of the shoulder upon weight bearing. This may be caused by lack of lateral collateral support (suprascapular muscles) or by additional involvement of the pectoral nerve, subscapular nerve, caudal cervical nerve roots, or other muscular and tendinous supporting structures of the shoulder. | Collision of shoulder with objects. |
| Radial | Unable to bear weight on affected limb because of a lack of elbow extension. The shoulder is rested in an extended position and the limb rests with the dorsum of the pastern on the ground. The limb may be moved forward by the action of the pectoral girdle muscles while the horse walks. | Often damaged in conjunction with humeral fracture; Occasionally observed after recovery from general anesthesia. |
| Brachial plexus | Signs of radial nerve paralysis often predominate. Evidence of suprascapular involvement not uncommon. Involvement of other nerves and nerve roots may be confirmed with EMG. Possible long-term atrophy of triceps. May have diffuse hypalgesia of lower limb. | Compression of the brachial plexus and radial nerve roots between the scapula and the ribs. |
| Musculocutaneous | Paralysis not common. Gait not markedly altered. Elbow may be overextended. Hypalgesia of medial forearm. Ultimately biceps and brachial muscles atrophy. | Uncommon. |
| Median and ulnar | Stiff, goose-stepping gait with hyperextension of the carpal, fetlock, and pastern joints. | Uncommon. |
| Femoral | Unable to support weight as a result of lack of stifle extension. At a walk, the limb is advanced only with difficulty and the stride is markedly shortened. The limb buckles due to stifle, hock, and fetlock flexion if the horse attempts to bear weight. Quadriceps muscle will atrophy after 10-14 days. Patellar reflex is absent. | External blow to the limb; occasionally observed after recovery from general anesthesia. |
| Sciatic | Poor limb flexion with stifle and hock extended and fetlock flexed when the horse is not bearing weight. Weight can be supported if the foot is extended; otherwise weight is supported on the dorsal surface of the foot. Limb hypalgesia from stifle downward with the exception of the medial surface between stifle and hock. | Variety of injuries may be causal. Occasionally occurs secondary to intramuscular injections, especially in foals; occasionally observed after recovery from general anesthesia. |
| Tibial | Hypermetric or stringhalt-like gait with flexion of the hock (dropped hock) at rest. Hypalgesia of areas of caudal limb distal to the hock including most of the caudal and medial coronary band area. | Uncommon. |
| Peroneal | Frequently a component of sciatic nerve injury. Inability to flex the hock and extend the digits. Acutely there is hyperextension of the hock and hyperflexion of the fetlock and interphalangeal joints causing the horse to drag the fetlock along the ground. Short protraction phase to the stride. If the foot is placed in a normal position, the limb may bear weight. Hypalgesia of the craniolateral portion of the limb from the level of the hock to the fetlock. | Accessible to injury as it passes across the lateral surface of the tibia. Injury seen in horses after recovery from general anesthesia or secondary to a kick or blow to the lateral side of the pelvic limb. |
Enzyme Determination
Increased CK activity also may suggest other diseases of the CNS. In one study, CK activity (>1 IU/L) most often was associated with EPM in horses and may be helpful in differentiating compressive spinal cord disease from EPM. Furthermore, persistently increased CSF CK activity may be associated with a poor prognosis in horses with EPM.19 Lactate dehydrogenase activity may be increased in spinal lymphosarcoma.
Lactic Acid Concentration
CSF lactic acid concentration may be an indicator of neurologic disease (see Table 12-2). CSF lactic acid concentration increases in eastern equine encephalomyelitis (4.10 ± 0.6 mg/dl), head trauma (5.40 ± 0.9 mg/dl), and brain abscess (4.53 mg/dl). Lactic acid concentration may be the only CSF parameter increased in horses with brain abscess.20
TABLE 12-2 Cerebrospinal Fluid Values from Atlantooccipital and Lumbosacral Spaces of Normal Healthy Adult Horses∗
| Atlantooccipital Space (Mean ± SD [Range]) | Lumbosacral Space (Mean ± SD [Range]) | |
|---|---|---|
| Red blood cell (RBC) count (per μl) | 51.0 ± 160 (0-558) | 36.8 ± 59.7 (0-167) |
| White blood cell count (per μl) | 0.33 ± 0.49 (0-1) | 0.83 ± 1.11 (0-3) |
| Total protein (mg/dl)∗ | 87.0 ±± 17.0 (53 ± 11.6) | 93.0 ± 16.0 (58.0 ± 11.0) |
| (59-118) (35-74) | (65-124) (39-78) | |
| Albumin (mg/dl) | 35.8 ± 9.7 (24-51) | 37.8 ± 11.2 (24-56) |
| Albumin quotient (AQ) | 1.4 ± 0.4 (1.0-2.0) | 1.5 ± 0.4 (1.0-2.0) |
| Immunoglobulin G (IgG) (mg/dl) | 5.6 ± 1.4 (3-8) | 6.0 ± 2.1 (3-10) |
| IgG index | 0.19 ± 0.046 (0.12-0.27) | 0.19 ± 0.5 (0.12-0.26) |
| Creatine kinase (CK) (IU/L) | 0-8 | 0-8 |
| Lactate dehydrogenase (IU/L) | 0-8 | 0-8 |
| Aspartate aminotransferase (AST) (IU/L) | 4-16 | 0-16 |
| Glucose (mg/dl) | 35%-70% of blood glucose | |
| Lactate (mg/dl) | 1.92 ± 0.12 | 2.30 ± 0.21 |
| Sodium (mEq/L) | 140-150 | 140-150 |
| Potassium (mEq/L) | 2.5-3.5 | 2.5-3.5 |
∗ Total protein concentration next to the mean ± SD in parentheses is the value expected using a total protein standard.
Electromyography
Needle EMG is the graphic recording of muscle cell electric activity during contraction or at rest from a recording electrode placed in the muscle. Electric activity is recorded by means of an amplifier on an oscilloscope.1–3 Nerve conduction studies consist of stimulating a peripheral nerve with electric current and recording the resultant physiologic electric activity from other segments of the nerve or from the muscles innervated by those nerves.4 Together, EMG and nerve conduction studies may help in the localization, diagnosis, and prognosis of diseases of the lower motor unit. The motor unit consists of the ventral horn cell bodies (located in the ventral horn of the spinal cord), its axon, the peripheral nerve, the myoneural junction, and the muscle fibers it innervates (Figure 12-3).
Electromyographic Examination
In the needle EMG examination, one should thrust the exploring electrode briskly into the muscle and hold it until the animal completely relaxes. To enable relaxation, one can force the animal to bear weight on the opposite limb. Once relaxation has occurred, one can evaluate the resting activity or any postinsertional activity of the muscle. One should evaluate at least four areas and depths of smaller skeletal muscles and six to eight areas and depths of larger skeletal muscles when possible. One should examine the horse systematically so as not to miss a lesion. Needle EMGs can also be performed in many of the major extrinsic muscles of the horse (Table 12-3). In addition, they can be performed on facial, laryngeal, esophageal, pectoral, and external anal sphincter muscles when indicated by neurologic examination. One may perform nerve conduction studies and collect muscle biopsy specimens to define suspected lesions further or confirm a diagnosis.
TABLE 12-3 Muscles, Nerves, and Nerve Roots Evaluated during Routine Electromyographic Examination of Horses
| Muscles | Peripheral Nerve | Spinal Nerve Root |
|---|---|---|
| REAR LIMB | ||
| Long digital extensor | Peroneal nerve | L6-S1 |
| Gastrocnemius | Tibial nerve | S1-S2 |
| Deep digital flexor | Tibial nerve | S1-S2 |
| Semimembranosus | Ischiatic nerve | L5-S2 |
| Vastus lateralis | Femoral nerve | L3-L5 |
| Biceps femoris | Caudal gluteal, ischiatic, and peroneal nerves | L6-S2 |
| Middle gluteal | Cranial and caudal gluteal nerves | L5-S2 |
| PARAVERTEBRAL | ||
| Paravertebral muscles (segmentally) | Dorsal branches of ventral spinal nerves (L6-C1) | L6-C1 |
| THORACIC LIMB | ||
| Subclavius | Pectoral nerve | C6-C7, T1 |
| Supraspinatus | Suprascapular nerve | C6-C8 |
| Infraspinatus | ||
| Deltoideus | Axillary nerve | C6-C8 |
| Biceps brachii | Musculocutaneous nerve | C6-C8 |
| Triceps | Radial nerve | C7-T1 |
| Extensor carpi radials | Radial nerve | C7-T1 |
| Superficial digital flexor | Ulnar nerve | C8-T2 |
| Deep digital flexor | Ulnar and median nerve | C7-T1, T2 |
Nerve Conduction Studies
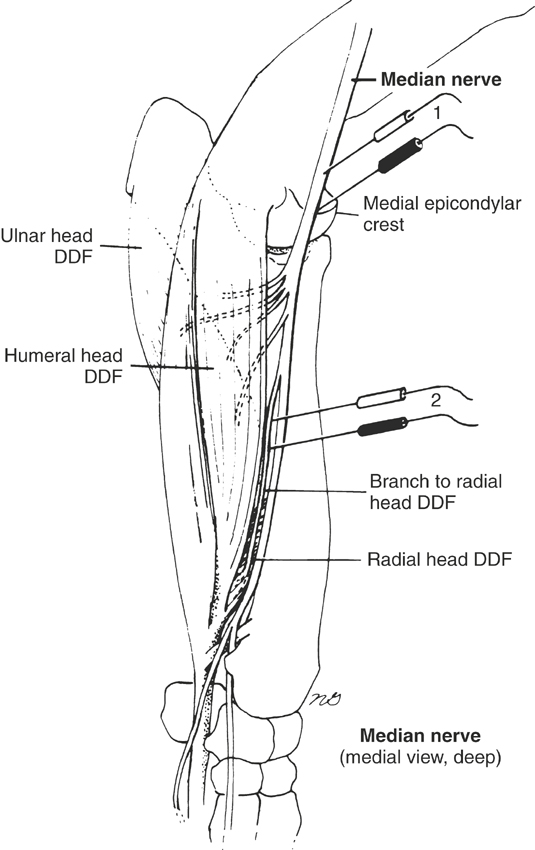
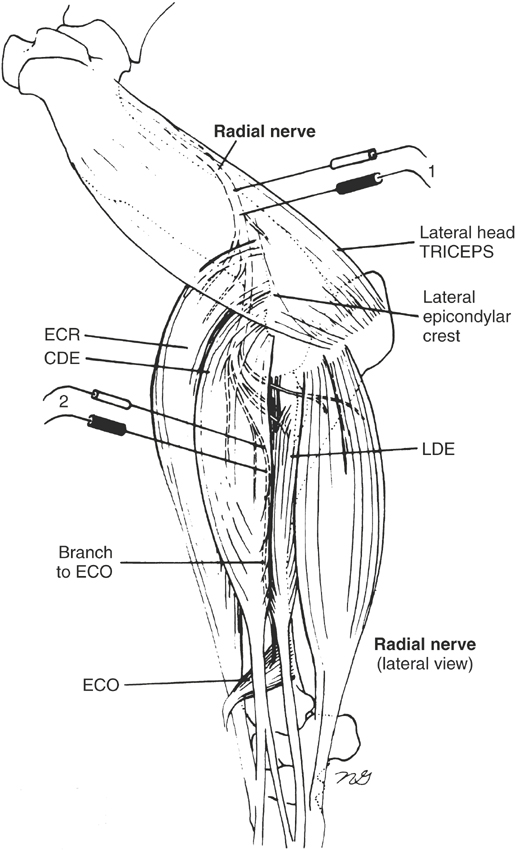
Nerve conduction studies are difficult to do in horses and therefore are not done routinely. However, the technique for radial and median nerve conduction studies in the horse has been reported.5,6 One must perform nerve conduction studies with the horse under general anesthesia and may use them to evaluate the speed of conduction of large myelinated motor nerves. To do this, the horse should be placed in lateral recumbency, with the affected side up. One stimulates the appropriate motor nerve by monopolar needle electrodes inserted at or near the nerve and records an evoked MUAP from an innervated muscle (Figures 12-4 and 12-5). The examiner may see or palpate the contraction of appropriate muscles and insert needle electrodes until a repeatable response is obtained. The unaffected limb can be used as a control.
In horses, one can obtain radial nerve recordings from the extensor carpi radialis and abductor digiti longus (extensor carpi obliquus) muscles (see Figure 12-4) and can obtain median nerve recordings from the humeral and radial heads of the deep digital flexor tendon5,6 (see Figure 12-5). Facial nerve recordings can be obtained from the levator nasolabialis muscle by stimulating the buccal branch of the facial nerve just ventral to the facial crest.7 Often a supramaximal stimulus can be obtained at 70 to 90 V for 0.1-ms duration. Nerve conduction studies are helpful in diagnosing radial nerve, median nerve, and possibly facial nerve injury.
Normal Electromyographic Potentials
Insertional Activity
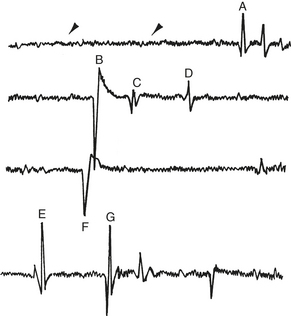
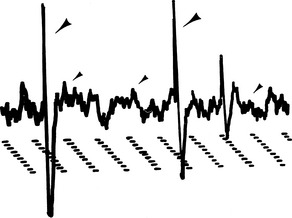
Insertional activity consists of short bursts of high-amplitude, moderate- to high-frequency (<200 Hz) electric activity after insertion or movement of the exploring electrode in the muscle (Figures 12-6 and 12-7). In normal skeletal muscle this activity stops a few milliseconds after cessation of needle movement. Insertional activity may be caused by mechanical stimulation, muscle fiber injury,3,8,9 or depolarization of muscle fibers directly adjacent to the EMG needle.1 Positive sharp waves and fibrillation potentials observed during (or associated with) needle insertion that stop after cessation of needle movement are considered normal. Damage to muscle fibers by needle insertion is probably the source of these potentials. However, positive sharp waves and fibrillation potentials persisting after needle insertion are considered abnormal and may suggest early muscle denervation.3
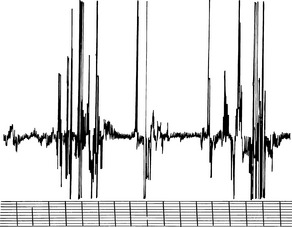
FIGURE 12-6 Normal insertional activity in the infraspinatus muscle. Gain: 0.500 mV/division; time: 10 ms/division.
Resting Activity (Postinsertional Baseline)
Resting activity is observed in relaxed muscle and is characterized by electric silence. A flat line appears on the oscilloscope. When the needle comes to rest near a nerve twig or end plate, the needle may irritate small intramuscular nerve terminals, which results in the production of two characteristic potentials: (1) end plate noise and (2) end plate spikes. End plate noise produces a rippling of the baseline and a low-pitched continuous noise (Figure 12-8). End plate spikes, on the other hand, are high-amplitude intermittent spikes and make a popping sound. End plate noise and spikes can occur alone or together. The origin of end plate noise is thought to be extracellularly recorded miniature end plate potentials,10,11 whereas end plate spikes are thought to be single muscle fiber contractions after needle electrode irritation of the nerve terminals.11 In human beings, these potentials are associated with dull pain3; repositioning the needle often eliminates their activity.
Motor Unit Action Potentials
Motor unit action potentials (MUAPs) are voluntary or reflex muscle contractions observed after insertion of the needle electrode. They represent the sum of a number of single muscle fiber potentials belonging to the same motor unit. MUAPs are usually monophasic, biphasic, and triphasic. Because individual muscle fibers fire nearly synchronously, the prefixes refer to the number of phases above and below the baseline (see Figure 12-7). A few polyphasic potentials (greater than four phases) may occur in normal muscle but usually do not exceed 5% to 15% of the population of MUAPs observed.3 MUAPs have an amplitude ranging between 500 to 3000 μV and a duration ranging between 1 to 15 ms. Examination of the conscious horse enhances the evaluation of the amplitude and number of phases of MUAPs in the muscle.
One may see these MUAPs when one forces the animal to bear weight on or retract a limb, resulting in contraction of that explored muscle. In lightly stimulated muscle, one may see single MUAPs, because single motor units are recruited (see Figure 12-7). As muscle contraction becomes more intense, more motor units are recruited and the greater frequency of MUAPs appears on the oscilloscope. Once MUAPs fill the screen, one observes an interference pattern. Clinically, the number of phases and the duration of MUAPs are of greater importance than amplitude, because amplitude may be influenced by species, the muscle explored, the age of the horse, and electrode position.9 Furthermore, MUAP duration has been shown to increase with age in human beings.12
Evoked Muscle Potentials
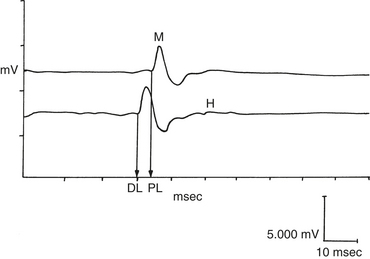
Stimulation of a mixed motor and sensory nerve results in two observed potentials: (1) the direct evoked muscle action potential (M wave) and (2) the reflex evoked muscle action potential (H wave). The M wave is the direct muscle action potential resulting from orthodromic conduction of direct nerve stimulation (Figure 12-9). The M wave is the most commonly used potential in veterinary medicine. This wave is usually biphasic or triphasic and is larger than the H wave. The amplitude of these evoked potentials depends on the number of motor units activated and on the size of the muscle, predominant fiber type, and type of recording electrode used.4 With monopolar electrodes, amplitudes observed in the horse range between 5 to 60 mV for a 2- to 10-ms duration. Normal amplitudes and durations have been reported for several muscles in the horse. The time required for the potential to travel down the motor nerve, cross the neuromuscular junction, travel down the muscle membrane, and stimulate a response in the muscle is called the latency. When two points along the motor nerve are stimulated, one can measure the distance between the stimulating electrodes (in millimeters) and divide it by the difference in latencies (in milliseconds). Normal median and radial nerve conduction velocities are 60 to 80 m/sec.5,6 Normal facial nerve conduction velocities are 55 to 70 m/sec.7

 . A head tilt is characterized by the poll deviated about the muzzle and must be distinguished from abnormal or unusual turning of the head or lateral deviation of the head as may occur with damage to the forebrain or injury to the cervical vertebrae. Additional signs that may accompany vestibular disease or injury include nystagmus, ipsilateral weakness, and facial nerve paralysis.
. A head tilt is characterized by the poll deviated about the muzzle and must be distinguished from abnormal or unusual turning of the head or lateral deviation of the head as may occur with damage to the forebrain or injury to the cervical vertebrae. Additional signs that may accompany vestibular disease or injury include nystagmus, ipsilateral weakness, and facial nerve paralysis. the brainstem along the medial longitudinal fasciculus. Deviations of the eyes may occur with head trauma or midbrain lesions or can be normal variations in newborn foals. When the head is elevated dorsally, the eyes should move ventrally to maintain a normal horizontal gaze. When the head is moved from side to side, the eyes should move slowly opposite to the direction of the head movement, then move quickly in the direction of the head movement; this is referred to as normal vestibular nystagmus. Spontaneous or positional nystagmus is always abnormal.
the brainstem along the medial longitudinal fasciculus. Deviations of the eyes may occur with head trauma or midbrain lesions or can be normal variations in newborn foals. When the head is elevated dorsally, the eyes should move ventrally to maintain a normal horizontal gaze. When the head is moved from side to side, the eyes should move slowly opposite to the direction of the head movement, then move quickly in the direction of the head movement; this is referred to as normal vestibular nystagmus. Spontaneous or positional nystagmus is always abnormal. The nuclei along the fifth (trigeminal) cranial nerve are among the largest nuclei along the brainstem of the horse. The fifth cranial nerve contains motor and sensory branches that supply innervation to the muscles of mastication and sensation to the skin and mucous membranes of the head. Injury to this nerve leads to dropped jaw and ipsilateral loss of, or decreased sensation to, the side of the face and the inside of the nares.
The nuclei along the fifth (trigeminal) cranial nerve are among the largest nuclei along the brainstem of the horse. The fifth cranial nerve contains motor and sensory branches that supply innervation to the muscles of mastication and sensation to the skin and mucous membranes of the head. Injury to this nerve leads to dropped jaw and ipsilateral loss of, or decreased sensation to, the side of the face and the inside of the nares. Damage to the seventh (facial) and eighth cranial nerves is common in horses. Injury to the seventh cranial nerve results in unilateral facial paralysis. This nerve contains branches that supply the ears, eyelids, and nares, and so injury to this nerve may affect all or only part of these structures. The most easily recognized sign is deviation of the nares toward the unaffected side coupled with drooping of the eyelid and ear on the affected side. Because this nerve also innervates the salivary and lacrimal glands, loss of or damage to this nerve may cause dry eye and decreased salivation.
Damage to the seventh (facial) and eighth cranial nerves is common in horses. Injury to the seventh cranial nerve results in unilateral facial paralysis. This nerve contains branches that supply the ears, eyelids, and nares, and so injury to this nerve may affect all or only part of these structures. The most easily recognized sign is deviation of the nares toward the unaffected side coupled with drooping of the eyelid and ear on the affected side. Because this nerve also innervates the salivary and lacrimal glands, loss of or damage to this nerve may cause dry eye and decreased salivation. Eighth cranial nerve deficits are easy to recognize, because unilateral injury to this nerve results in a head tilt toward the affected side. The eighth cranial nerve is important to hearing and control of balance. Projections from this nerve pass to the medulla and cerebellum. A horse that has damage to this nerve often appears disoriented and has a head tilt toward the side of the lesion along with abnormal position of the limbs and body and horizontal nystagmus. If the lesion involves the peripheral portion of the vestibular system, then the fast phase of the nystagmus is directed away from the side of the lesion. If the lesion involves the central portion of the vestibular system, then the nystagmus may appear vertical, rotary, or horizontal and may not always appear the same, depending on head position.
Eighth cranial nerve deficits are easy to recognize, because unilateral injury to this nerve results in a head tilt toward the affected side. The eighth cranial nerve is important to hearing and control of balance. Projections from this nerve pass to the medulla and cerebellum. A horse that has damage to this nerve often appears disoriented and has a head tilt toward the side of the lesion along with abnormal position of the limbs and body and horizontal nystagmus. If the lesion involves the peripheral portion of the vestibular system, then the fast phase of the nystagmus is directed away from the side of the lesion. If the lesion involves the central portion of the vestibular system, then the nystagmus may appear vertical, rotary, or horizontal and may not always appear the same, depending on head position.
 stimulation the horse may relax and eventually raise its tail.
stimulation the horse may relax and eventually raise its tail. To evaluate the gait of an animal, one begins by observing the horse at a walk and trot. At a walk the examiner can walk alongside, in step with first the pelvic limbs and then the thoracic limbs. This allows the examiner to more easily determine the stride length and foot placement. A weak limb often has a low arc and longer stride length.
To evaluate the gait of an animal, one begins by observing the horse at a walk and trot. At a walk the examiner can walk alongside, in step with first the pelvic limbs and then the thoracic limbs. This allows the examiner to more easily determine the stride length and foot placement. A weak limb often has a low arc and longer stride length. One also should observe the horse walking in a circle, on a slope, and with its head elevated. These procedures provide a degree of “challenge” to the horse and may help to demonstrate if the horse is showing persistent irregular movements with its limbs. Horses with a musculoskeletal problem have been described as being a regularly irregular gait, whereas a horse with neurologic gait deficits shows less consistency placing its limbs (an irregularly irregular gait). Although observing the horse running free in a paddock or round pen and while being ridden can sometimes be helpful, this is not always possible. One must consider safety for the horse and rider, as well as certain legal ramifications before asking a person to ride a horse during the examination.
One also should observe the horse walking in a circle, on a slope, and with its head elevated. These procedures provide a degree of “challenge” to the horse and may help to demonstrate if the horse is showing persistent irregular movements with its limbs. Horses with a musculoskeletal problem have been described as being a regularly irregular gait, whereas a horse with neurologic gait deficits shows less consistency placing its limbs (an irregularly irregular gait). Although observing the horse running free in a paddock or round pen and while being ridden can sometimes be helpful, this is not always possible. One must consider safety for the horse and rider, as well as certain legal ramifications before asking a person to ride a horse during the examination. Additional tests that may be helpful during a neurologic examination include blindfolding the horse, walking it over a curb or other obstacle, and walking it with its head and neck extended. One can evaluate the strength and ability of the horse to correct body positions by performing a sway test by applying lateral pressure at the shoulder, hip, and tail while the horse is standing and walking. One should apply pressure several times while the horse is walking to catch the limb in various stages of weight bearing. Observing a horse during the backing process is important. When a normal horse is backing, it should lift each leg and place it in a coordinated and appropriate location. Horses with neurologic abnormalities often place the limbs in wide-based positions or lean back and are reluctant or refuse to move. The horse also may step on the feet of a pelvic limb with the front feet.
Additional tests that may be helpful during a neurologic examination include blindfolding the horse, walking it over a curb or other obstacle, and walking it with its head and neck extended. One can evaluate the strength and ability of the horse to correct body positions by performing a sway test by applying lateral pressure at the shoulder, hip, and tail while the horse is standing and walking. One should apply pressure several times while the horse is walking to catch the limb in various stages of weight bearing. Observing a horse during the backing process is important. When a normal horse is backing, it should lift each leg and place it in a coordinated and appropriate location. Horses with neurologic abnormalities often place the limbs in wide-based positions or lean back and are reluctant or refuse to move. The horse also may step on the feet of a pelvic limb with the front feet. The horse should be observed closely while being turned in a tight circle to identify abnormal wide outward excursions of the pelvic limb (circumduction). Additional tests to assess proprioceptive function include a standing sway test in which one applies pressure to the shoulder. The horse initially should press into the examiner and then lean away, and finally it should step away with the offside limb. The author also crosses the limbs over the opposite thoracic or pelvic limb to determine if the horse recognizes and tolerates these unusual limb positions. After this, the examiner might lift one thoracic limb and force the horse to hop on the opposite limb in a modified postural reaction.
The horse should be observed closely while being turned in a tight circle to identify abnormal wide outward excursions of the pelvic limb (circumduction). Additional tests to assess proprioceptive function include a standing sway test in which one applies pressure to the shoulder. The horse initially should press into the examiner and then lean away, and finally it should step away with the offside limb. The author also crosses the limbs over the opposite thoracic or pelvic limb to determine if the horse recognizes and tolerates these unusual limb positions. After this, the examiner might lift one thoracic limb and force the horse to hop on the opposite limb in a modified postural reaction. Weakness or paresis is a deficiency of voluntary movement as a result of loss of muscle power because of either damage to upper motor neurons, lower motor neurons, or the muscle. It can be described as knuckling, stumbling, or buckling and sometimes can be characterized by toe-dragging while walking. Weakness may be associated with an upper motor neuron or lower motor neuron lesion. In the case of a lower motor neuron or peripheral nerve injury or illness, the horse shows muscle atrophy and sensory loss. A spastic movement of the limbs often accompanies the weakness that results from loss of upper motor neurons. Ataxia is typified by abnormal foot placement and wide swaying of the foot and limb, especially while turning. Ataxia appears as a lack of coordination of motor movements. It can arise from damage to the vestibular, cerebellar, or sensory portions of the nervous system. Ataxia in horses is most often a result of loss of sensory input through injury, damage, or disease in the spinal cord blocking normal input to the cerebellum. Cerebellar ataxia is seen most often in young horses, typically Arabians with a condition known as cerebellar abiotrophy. Vestibular ataxia is often accompanied by a head tilt and other cranial nerve deficits.
Weakness or paresis is a deficiency of voluntary movement as a result of loss of muscle power because of either damage to upper motor neurons, lower motor neurons, or the muscle. It can be described as knuckling, stumbling, or buckling and sometimes can be characterized by toe-dragging while walking. Weakness may be associated with an upper motor neuron or lower motor neuron lesion. In the case of a lower motor neuron or peripheral nerve injury or illness, the horse shows muscle atrophy and sensory loss. A spastic movement of the limbs often accompanies the weakness that results from loss of upper motor neurons. Ataxia is typified by abnormal foot placement and wide swaying of the foot and limb, especially while turning. Ataxia appears as a lack of coordination of motor movements. It can arise from damage to the vestibular, cerebellar, or sensory portions of the nervous system. Ataxia in horses is most often a result of loss of sensory input through injury, damage, or disease in the spinal cord blocking normal input to the cerebellum. Cerebellar ataxia is seen most often in young horses, typically Arabians with a condition known as cerebellar abiotrophy. Vestibular ataxia is often accompanied by a head tilt and other cranial nerve deficits. Abnormal gaits associated with stringhalt, upward fixation of the patella, and fibrotic myopathy deserve careful attention. Horses that show these gait abnormalities have a mechanical lameness, although the exact cause is unknown and may sometimes be associated with underlying neurologic disease.
Abnormal gaits associated with stringhalt, upward fixation of the patella, and fibrotic myopathy deserve careful attention. Horses that show these gait abnormalities have a mechanical lameness, although the exact cause is unknown and may sometimes be associated with underlying neurologic disease. Fibrotic myopathy results from scar tissue formation after injury to the semitendinosus and semimembranosus muscles. One may confuse the characteristic foot placement coupled with the abrupt rearward movement of the affected limb with a spastic gait caused by spinal cord injury (SCI) or disease. With careful examination, a horse suffering from fibrotic myopathy likely will not go undiagnosed.
Fibrotic myopathy results from scar tissue formation after injury to the semitendinosus and semimembranosus muscles. One may confuse the characteristic foot placement coupled with the abrupt rearward movement of the affected limb with a spastic gait caused by spinal cord injury (SCI) or disease. With careful examination, a horse suffering from fibrotic myopathy likely will not go undiagnosed. Horses with profound ataxia may pace when they walk, which often is accompanied by circumduction of the outside rear limb while turning. These signs suggest general proprioceptive deficits. If the horse has these deficits while walking, then the examiner should observe the horse walking with its head elevated and on a slope (these maneuvers often exaggerate a subtle problem).
Horses with profound ataxia may pace when they walk, which often is accompanied by circumduction of the outside rear limb while turning. These signs suggest general proprioceptive deficits. If the horse has these deficits while walking, then the examiner should observe the horse walking with its head elevated and on a slope (these maneuvers often exaggerate a subtle problem). If the horse shows signs only in the trunk and pelvic limbs, the neuroanatomic localization of the lesion is between T2 and S2 or involves the nerves and muscles of the pelvic limbs. However, horses with cervical spinal cord lesions often demonstrate signs in the pelvic limbs that are one grade worse than those observed in the thoracic limbs. Therefore a mild cervical spinal cord or brainstem lesion could show minimal or no thoracic limb signs with grade 1 or subtle signs in the pelvic limbs.
If the horse shows signs only in the trunk and pelvic limbs, the neuroanatomic localization of the lesion is between T2 and S2 or involves the nerves and muscles of the pelvic limbs. However, horses with cervical spinal cord lesions often demonstrate signs in the pelvic limbs that are one grade worse than those observed in the thoracic limbs. Therefore a mild cervical spinal cord or brainstem lesion could show minimal or no thoracic limb signs with grade 1 or subtle signs in the pelvic limbs. Palpating the horse over its back, rump, and muscles of the rear limbs while being careful to detect any muscle atrophy is helpful. The author routinely stimulates the horse over the side of its body and observes for any twitching of the cutaneous trunci muscles. Such twitching usually is accompanied by a cerebral response and requires a fairly severe lesion to detect areas of analgesia.
Palpating the horse over its back, rump, and muscles of the rear limbs while being careful to detect any muscle atrophy is helpful. The author routinely stimulates the horse over the side of its body and observes for any twitching of the cutaneous trunci muscles. Such twitching usually is accompanied by a cerebral response and requires a fairly severe lesion to detect areas of analgesia. To complete the neurologic examination, one must examine the tail and anus to determine whether damage has occurred to the sacrococcygeal nerve and muscle segments. A normal perineal reflex results in contraction of the anus and clamping of the tail in response to light stimulation of the skin in this region. Cauda equina neuritis or polyneuritis equi, trauma, and iatrogenic injury resulting from an alcohol tail block are some disorders that may affect this area.
To complete the neurologic examination, one must examine the tail and anus to determine whether damage has occurred to the sacrococcygeal nerve and muscle segments. A normal perineal reflex results in contraction of the anus and clamping of the tail in response to light stimulation of the skin in this region. Cauda equina neuritis or polyneuritis equi, trauma, and iatrogenic injury resulting from an alcohol tail block are some disorders that may affect this area.