CHAPTER 15 Disorders of the Gastrointestinal System∗
PHYSICAL EXAMINATION
Abdominal auscultation is particularly useful for assessing the motility of the large intestine. Progressive motility of the small intestine, conversely, is difficult to distinguish by auscultation from nonprogressive motility. The distinct character of the borborygmi produced during propulsive contractions of the cecum and ascending colon allows evaluation of the frequency and strength of retropulsion and propulsion. Propulsive contractions of the cecum and ventral colon occur every 3 to 4 minutes and give rise to prolonged rushing sounds heard over long segments of intestine. Retropulsive sounds presumably are similar to propulsive sounds, but they occur less frequently. Distinguishing between propulsion and retropulsion is not important clinically because both types of contractions signify normal motility. Interhaustral and intrahaustral mixing contractions produce nonspecific sounds of fluid and ingesta movement that are difficult to distinguish from other borborygmi, such as small intestinal contractions or spasmodic contractions.1
Auscultation over the right flank and proceeding along the caudal edge of the costal margin toward the xiphoid allows evaluation of the cecal borborygmi. Auscultation over a similar area on the left side allows evaluation of the pelvic flexure and ascending colon. Typical progressive borborygmi heard every 3 to 4 minutes on both sides of the abdomen indicate normal motility of the cecum and ascending colon. Less frequent progressive sounds may indicate a pathologic condition of the large intestine or may result from anorexia, nervousness (sympathetic tone), or pharmacologic inhibition of motility (i.e., α2-adrenergic agonists such as xylazine).2–5 Absolute absence of any auscultable borborygmi suggests abnormal motility and indicates ileus resulting from a serious pathologic condition but is not specific to any segment of the intestine.3,6 If borborygmi are audible but progressive sounds are not detectable, determining whether a significant abnormality exists is difficult.6 Borborygmi heard more frequently than normal may result from increased motility following feeding; from excessive stimulation from irritation, distention, or inflammation; or after administration of parasympathomimetic drugs such as neostigmine. Large intestinal motility increases in the early stages of intestinal distention regardless of the site.7 Mild inflammation or irritation of the large intestinal mucosa also can stimulate motility.3 Parasympathomimetic drugs stimulate contractions and auscultable borborygmi in the large intestine; however, an increase in parasympathetic tone may result in segmental contractions, which actually inhibit progressive motility.2
One can detect sand or gravel in the large intestinal ingesta by auscultation behind the xiphoid process. It is possible to hear sand or gravel particles grinding together during progressive contractions of the ascending colon. In one study characteristic sounds were heard on auscultation (5 minutes’ duration) after daily administration of sand to healthy horses, none of which developed clinical signs of colic or diarrhea.8 If the frequency of progressive contractions is low or absent, detecting sand by auscultation is difficult.
Transrectal palpation is the most specific physical examination technique for investigation of intestinal disease and is particularly valuable when evaluating obstructive diseases.9,10 The primary objectives of transrectal palpation are to assess the size, consistency, and position of the segments of the large intestine; to determine the presence of any distention of the small intestine; and to detect intra-abdominal masses. Evaluation of the wall thickness and texture and the mesenteric structures (blood and lymphatic vessels and lymph nodes) also may aid in diagnosis of large intestinal disease. The interpretation of transrectal palpation findings in light of clinical signs and laboratory results is an important diagnostic aid for developing appropriate treatment strategies for intestinal diseases manifested by abdominal pain. Enlargement of one or more segments of large intestine detected by transrectal palpation provides evidence of obstruction at or distal to the enlarged segment. By systematically evaluating each segment, one can determine the site of obstruction. Obstruction of the pelvic flexure, for instance, results in enlargement of the pelvic flexure and ventral colon, but the dorsal and descending colons are of normal size. Enlargement of a segment of the large intestine usually is accompanied by abnormal consistency of the contents. It is possible to distinguish among gas, fluid, and ingesta and to detect foreign bodies in palpable segments. Accumulation of gas and fluid suggests complete and acute obstruction, whereas accumulation of ingesta suggests chronic and incomplete obstruction. Accumulation of fluid usually indicates ileus. The practitioner must evaluate the consistency of the contents in light of the size of the segment; ingesta in the ventral colon of a dehydrated patient may be firm, but the size of the ventral colon will be normal. Conversely, if the ingesta is firm because of a distal obstruction, the ventral colon will be enlarged.
Nonstrangulating causes of small intestinal distention can be divided further into intraluminal and extraluminal obstructions. Ileal impactions are probably the most common cause of intraluminal obstruction, and on rare occasions the impaction can be palpated in the upper right quadrant, near the ileocecal opening. Intraluminal masses caused by lymphoma, eosinophilic enteritis, foreign bodies, or ascarid impactions often lead to small intestinal distention and are usually indistinguishable from one another on the basis of palpation alone. Small intestine in these cases can be moderately to severely distended, depending on the degree of obstruction. Extraluminal obstructions include abdominal masses, abscesses or tumors, and large colon displacement. One should always palpate the rest of the abdomen carefully to help rule out these causes. Some cases of small intestinal distention result from a physiologic rather than a mechanical obstruction. Ileus may result postoperatively or after inflammatory diseases of the bowel (proximal enteritis) or peritoneal cavity (peritonitis). The bowel is usually mildly to moderately distended and almost always is accompanied by significant amounts of accumulated gastric fluid.
DIAGNOSTIC EVALUATION
Clinical Pathology
Evaluation of the hemogram is essential when assessing conditions of the gastrointestinal tract. However, hematologic alterations associated with diseases of the gastrointestinal tract are often nonspecific, reflecting systemic response to inflammation, endotoxemia, or sepsis. Neutrophilic leukocytosis and normochromic, normocytic anemia with or without hyperfibrinogenemia commonly are associated with chronic inflammatory conditions of the intestine. Anemia from chronic blood loss occurs infrequently in adult horses because of the large iron stores and high concentrations of iron in their diet; anemia usually follows chronic inflammation, as do alterations in the leukon and plasma fibrinogen concentrations. Plasma protein concentrations vary depending on gastrointestinal losses of albumin and globulin and elevation of globulin concentration from antigenic stimulation. Protein-losing enteropathies may manifest predominantly as a hypoalbuminemia or as a panhypoproteinemia. Immunoglobulin quantification can be useful in selected cases; immunosuppression with low immunoglobulin M concentration has been shown to occur in some cases of lymphosarcoma.11 Parasitic infections, especially strongylosis, may be characterized by elevated serum immunoglobulin G(T) concentration.12
Significant alterations of the hemogram do not accompany acute disease of the intestine unless severe inflammation, dehydration, endotoxemia, or sepsis is present. During the early stages of endotoxemia, elevations in circulating concentrations of inflammatory mediators, epinephrine, and cortisol produce characteristic changes in the hemogram. Leukopenia, with neutropenia and a left shift, toxic changes in the neutrophil cytoplasm, and lymphopenia commonly occur.13 Hemoconcentration and hyperfibrinogenemia are also common. Thrombocytopenia and other coagulopathies are also features of endotoxemia. Indeed, thrombocytopenia may be the earliest indicator of sepsis.14 Endotoxemia and circulating mediators of inflammation activate the coagulation cascade, causing a hypercoagulable state that can lead to consumption of coagulation factors and coagulation defects manifested as elevated prothrombin time, partial thromboplastin time, fibrin degradation products, and bleeding time and reduced activity of antithrombin III.15–17 Neutrophilic leukocytosis occurs during the later stages of endotoxemia.15
The most common serum biochemical abnormalities with diseases of the large or small intestine are electrolyte imbalances. Serum calcium concentrations are often low with strangulating obstructions and acute inflammatory diseases.18 Inflammation of the mucosa can severely disrupt electrolyte fluxes. Diarrhea or gastric reflux greatly exacerbates the loss of sodium, potassium, calcium, magnesium, and bicarbonate. Hypoxia and cellular damage caused by ischemia of the intestine may be reflected by an elevated serum phosphate concentration resulting from phosphate leakage from damaged cells.19 Ischemia and cellular hypoxia in any segment of the intestine also causes a shift in energy metabolism to anaerobic glycolysis, resulting in increased production of lactate and elevated serum lactate concentration. Reduced perfusion of peripheral tissues from hypotensive shock and intestinal ischemia can cause elevations in serum lactate. However, obstruction of the intestine during ischemia may result in absorption of lactate from the lumen.20,21 Anion gap is an indirect measurement of organic acid production during states of tissue hypoxia and is a reasonable estimate of serum lactate concentration.21 Metabolic acidosis may accompany lactic acidemia, but an inconsistent association exists between the two, especially when mixed acid-base imbalances are present.21,22 Elevations of hepatic enzymes, specifically γ-glutamyltransferase, may occur with large colon displacements, duodenal strictures, or anterior enteritis. An elevated γ-glutamyltransferase is more suggestive of a right dorsal displacement than a left dorsal displacement.23
Relative polycythemia from hemoconcentration or splenic contraction and changes in red blood cell deformability from hypoxia or hypocalcemia may increase blood viscosity. Blood viscosity increases in patients with acute obstructive disease. Hyperviscosity reduces perfusion of capillary beds, thereby exacerbating ischemia and tissue hypoxia.24 Hyperviscosity is one manifestation (along with lactic acidemia, coagulopathies, and clinical signs of shock) of the pathophysiologic events that take place during acute inflammatory or vascular injury to the large intestine. Laboratory tests designed to reflect the systemic effects of endotoxemia, ischemia, sepsis, and shock are important to design therapeutic strategies and monitor response to therapy.
PERITONEAL FLUID
PF reflects a sequence of events that takes place during acute vascular injury to the intestine. The PF protein concentration first increases, followed by an increase in the red blood cell count and fibrinogen concentration. A transudative process resulting from vascular congestion and increased endothelial permeability allows small macromolecules (albumin) to escape into the PF, followed by larger macromolecules (globulin and fibrinogen), and finally diapedesis of cells (red blood cells, and then white blood cells).25,26 If ischemic inflammation of the intestine and visceral peritonitis occurs, an exudative process ensues. Severe inflammation of the intestine and visceral peritoneum causes large quantities of protein and white blood cells, primarily neutrophils, to escape into the PF.24,26 As damage to the bowel progresses, the protein concentration and red blood cell and white blood cell counts continue to rise. As the degree of irreversible damage to the intestine increases, the PF characteristics become more exudative.25,26 Eventually, bacteria begin to translocate across the intestinal wall and appear in the PF as the mucosal barrier breaks down. Neutrophils predominate, their cytoplasm becomes granulated, and Döhle’s bodies often are visible. If perforation occurs, bacteria and particles of ingesta appear in the PF, and the neutrophils become degenerate (i.e., pyknotic), with karyorrhexis, karyolysis, and smudge cells.
Elevated PF protein concentration is a sensitive indicator of early inflammation, whereas elevated red blood cell counts in the presence of normal white blood cell counts suggest vascular damage without significant tissue ischemia.26 Of note, the anticoagulant potassium EDTA, but not lithium heparin, can cause an increase in total protein as measured by refractometer, relative to the value obtained from the same sample without anticoagulant.27 Elevation of the white blood cell count usually indicates severe tissue inflammation or intestinal injury.20 The gross color of the PF can be helpful in detecting injury and necrosis of the intestine. A serosanguinous appearance indicates vascular injury, whereas orange or brown-red indicates necrosis with the release of pigments such as hemosiderin. Serial samples of PF are most useful in determining the nature and extent of damage to the intestine, but in many cases of ischemia, irreversible tissue damage has occurred by the time PF abnormalities appear.
Tissue hypoxia and ischemia cause a rapid elevation of PF lactate dehydrogenase, creatine kinase, and alkaline phosphatase activity and lactate concentration.20,21,28,29 Phosphate concentration increases when cellular disruption occurs.19 PF enzyme activities, phosphate, and lactate concentration increase faster and higher than serum activities.19–21,28,29 PF pH and glucose concentration tend to decrease during intestinal ischemia but are not as low as in septic peritonitis.30 Although biochemical alterations may be early indicators of intestinal ischemia and necrosis, they are nonspecific and offer no advantage over conventional methods of PF analysis in many cases. PF alkaline phosphatase has been shown to arise predominantly from degenerating white blood cells, and elevations of other enzyme activities may occur with many inflammatory diseases.28 Thus the specificity of many tests performed on PF is questionable. However, in selected cases in which conventional PF analysis and physical examination do not provide sufficient information to develop a treatment plan, biochemical analysis of the PF may be useful.
Cytologically examined cells of the PF may reflect chronic inflammatory conditions of the large intestine, especially eosinophilic or lymphocytic processes.31 Infectious and inflammatory conditions often cause increases in the neutrophil count and may be indistinguishable unless bacteria are visible. One also may detect neoplastic diseases by PF examination. Chronic infection and inflammation may be associated with elevated PF protein and fibrinogen concentrations. Culture of PF usually is required to distinguish bacterial infections from noninfectious inflammation unless bacteria are visible on cytologic examination. However, culture of PF is often unrewarding because factors that are found in inflammatory PF inhibit bacterial growth, and leukocytes phagocytose many bacteria in the PF.32 Decreases in PF glucose concentrations (<30 mg/dl), and pH (<7.3) are early indicators of a septic process. The glucose concentration and pH in the PF should approximately equal the blood glucose concentration and pH. A PF fibrinogen concentration greater than 200 mg/dl also indicates bacterial infection.33
FECAL EXAMINATION
Gross examination of the feces can provide information about digestion and transit time in the large intestine. Large fiber particles in the feces represent poor mastication or poor digestion in the large intestine. Small, mucus-covered, hard fecal balls indicate prolonged transit through the descending colon, whereas increased fluidity implies decreased transit time. Feces containing sand or gravel are not necessarily abnormal. However, a significant amount of sand implies that large quantities are present in the colon. Frank blood indicates substantial bleeding into the distal colon (right dorsal colon, small colon, or both) resulting from mucosal damage.
Laboratory analysis of the feces is performed frequently in cases of diarrhea. Fecal cytologic examination and tests for occult blood detect mucosal inflammation, erosion, or ulceration. Severe inflammatory diseases in human beings, invasive bacterial infections in particular, have been shown to increase the shedding of leukocytes in the feces. A higher percentage of horses with salmonellosis and diarrhea have fecal leukocyte counts greater than 10 cells per high power field than horses with negative fecal cultures for Salmonella. These results suggest that high fecal leukocyte counts indicate salmonellosis in horses with diarrhea. However, the specificity of this test is probably low. Low fecal leukocyte counts do not rule out salmonellosis.34
Fecal occult blood tests detect blood in the feces, presumably from erosion or ulceration of the mucosa, but do not distinguish the source of the blood. Large volumes of blood (1 to 2 L) given by nasogastric tube were required to produce a positive test for occult blood in the feces, but the amount of blood originating from the large intestine required to produce a positive test is unknown. A positive test implies significant hemorrhage into the gastrointestinal tract. Newer, more sensitive tests detect not only occult blood but also degraded blood and may be useful to determine the site and quantity of blood loss.35 However, large-scale evaluation of these tests is as yet unavailable.
Bacteriologic examination of the fecal flora has been used to quantitate specific bacterial species in the feces of horses with diarrhea. Quantitation of clostridial species may be beneficial in diagnosing clostridial infection of the large intestine.36 Tests to detect clostridial toxins in intestinal contents or feces are important to determine whether clostridia cultured from the feces are causing disease. The most common bacterial pathogens isolated from the feces of horses are Salmonella sp. and Clostridium sp. The number of Salmonella organisms isolated from the feces of horses with clinical salmonellosis is usually higher than from horses with asymptomatic infections. However, the volume of feces in many cases of acute diarrhea is high, and the concentration of Salmonella organisms may be lower than would be expected, accounting for many false-negative fecal cultures. The sensitivity of fecal cultures for detecting Salmonella infection may be as low as 20%. Culture of five consecutive daily fecal samples is recommended to increase the sensitivity of the test. Because salmonellae are intracellular organisms, culture of rectal scrapings or a rectal biopsy sample, along with fecal material, may increase the sensitivity of culture for detecting Salmonella infection to 50%.37 One can perform a polymerase chain reaction assay on fecal samples to detect DNA from Salmonella sp. The polymerase chain reaction test is more sensitive than culture and is frequently positive in clinically normal horses that continuously shed small amounts of bacteria. Polymerase chain reaction or immunologic tests also may detect Clostridium perfringens and Clostridium difficile exotoxins in the feces.
Qualitative fecal examination is a technique to detect nematode and cestode ova, protozoan oocysts, parasitic larvae, and protozoan trophozoites. A direct smear of fecal material is a rapid method to screen feces for ova and oocysts, to detect parasite larvae and trophozoites, and to observe motility of ciliates and parasite larvae. Fecal flotation is a more sensitive technique for isolating and detecting ova and oocysts because the eggs are concentrated from the sample. Zinc sulfate and sucrose solutions often are used to concentrate less dense ova and oocysts. Zinc sulfate produces less distortion of trophozoites and larvae than sucrose solutions. Fecal sedimentation is particularly appropriate for ciliates, Giardia organisms, and trichomonads. Quantitative techniques such as the Cornell-McMaster method allow estimation of the number of eggs per gram of feces and are most appropriate in monitoring parasite control programs.38
Radiography
Survey radiography of the normal esophagus is usually unrewarding but may be useful in horses with esophageal obstructions to determine the extent and location of the obstruction. It is possible to detect foreign bodies or soft tissue masses and, in cases of esophageal rupture, free air and ingesta in the tissues surrounding the esophagus. Pneumomediastinum also may be observed.39 Thoracic radiographs may be necessary to detect intrathoracic esophageal obstructions, megaesophagus, or cranial mediastinal masses causing extraluminal obstruction. One may use barium swallows or double-contrast esophagrams after resolution of the obstruction to determine whether a stricture, diverticulum, or other underlying disorder is present.40 Barium sulfate is the usual contrast medium and can be administered orally by way of a dose syringe or nasogastric tube (50 to 100 ml of a 40% barium sulfate suspension or barium paste). Oral administration is preferred for evaluation of swallowing and lesions in the proximal esophagus. Administration of contrast using a nasogastric tube (preferably cuffed) allows for delivery of larger volumes of barium (up to 500 ml) but should be performed without sedation if possible. Administration of contrast material can be followed with air insufflation to create a double-contrast effect. If rupture of the esophagus is suspected or if the contrast material is likely to be aspirated, iodinated organic compounds in an aqueous solution should be used as contrast material.39 Contrast radiography may be the most definitive method for the diagnosis of primary megaesophagus or other functional disorders such as autonomic dysautonomia (grass sickness) affecting the esophagus.40 When interpreting esophageal radiographs, the veterinarian should take particular care if the horse is sedated. Acepromazine or detomidine administration causes esophageal dilation in normal horses, especially after passage of a nasogastric tube.41
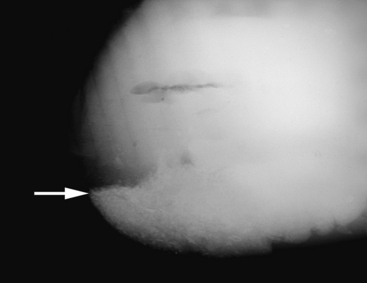
Radiography of the adult equine abdomen is an effective technique in detecting radiodense material in the large intestine, such as enteroliths, sand, and metallic objects.42,43 One survey demonstrated that radiography has 76.9% sensitivity and 94.4% specificity for diagnosing enterolithiasis.42 Radiography also can be a useful tool for detecting sand accumulation in the colon that causes diarrhea or impactions (Figure 15-1) and for monitoring resolution in medically treated horses.44 Recently, an objective scoring system demonstrated greater efficacy and less interobserver variability than a subjective assessment of radiographic sand accumulation in horses with or without a clinical diagnosis of sand colic.45 The large size and density of the adult abdomen precludes evaluation of soft tissue structures because the detail and contrast of the radiographs are usually poor. One is more likely to obtain diagnostically useful abdominal radiographs from small ponies and miniature horses than from full-size adult horses. Accumulation of gas is visible on radiographs of adult horses, but distinguishing normal intestinal gas from obstruction is often difficult. Horses should be fasted for 24 to 48 hours to reduce the amount of ingesta in the large intestine before radiography, if clinically warranted.
Abdominal radiography is more useful in foals than in adult horses. Radiographs are more detailed, and contrast can be good. Radiographic evidence of gas distention in the large intestine may indicate large intestinal obstruction, and radiographic signs of displacement are often diagnostic. Radiography allows the diagnosis of impactions, intussusceptions, foreign bodies, and other disorders. Functional ileus may be difficult to distinguish from mechanical obstruction.46,47 Administration of contrast (barium sulfate 30% at 5 ml/kg) through a nasogastric tube increases the diagnostic capabilities of radiography and is especially useful for diagnosis of gastric outflow obstruction in the older foal.48 Gastric ulceration also is recognizable with contrast radiography in the foal, although this is not as accurate a method as endoscopy.49 Contrast administered retrograde through a 24-F Foley catheter inserted into the rectum at a dose of up to 20 ml/kg has excellent potential for diagnosing disorders of the small colon, transverse colon, and large colon in foals.50
Ultrasonography
To evaluate the abdomen adequately, one must know the anatomic location and normal appearance of the individual organs. In the left cranial abdomen, it is possible to assess the greater curvature of the stomach between the eleventh and thirteenth intercostal space, and the spleen and the large splenic vein can be used as landmarks. Cases of gastric dilation from gas or impaction appear as an enlargement of the viewing area to cover more than five rib spaces.51 The veterinarian also can evaluate the stomach for intramural or extramural masses such as abscesses or for squamous cell carcinoma.52 The lesser curvature is not routinely visible. Assessment of the small intestine should include evaluation for changes in thickness, motility, location, and visibility. Small intestinal loops are easily found in the left lower quadrant of the abdomen, but these normally are visible in other locations. One can visualize the duodenum consistently on the right side of the abdomen deep to the liver in the tenth to twelfth intercostal space or deep to the right kidney at the fifteenth to sixteenth intercostal space. Mural thickening (>4 mm) may occur with edema, infiltrative or proliferative diseases, enteritis, and paralytic or mechanical ileus. Thickening of the small intestinal wall in neonatal foals, with or without the presence of intramural gas shadows, should raise suspicions of clostridial enteritis. One can assess motility by monitoring a specific area for contractions over time.
Ultrasonography can be an accurate method of distinguishing strangulating disorders of the small intestine from nonstrangulating disorders. Strangulated small intestine has thicker walls and a larger diameter than typically observed in nonstrangulating disorders. Strangulating lesions have decreased motility in the incarcerated segments, with normal motility elsewhere.53 Cases of paralytic ileus or nonstrangulating obstruction have a diffusely decreased peristalsis but not to the degree observed with strangulating lesions.51,53
Ultrasonography may be used to diagnose some specific lesions of the small intestinal tract. Ascarids may be visible in foals in cases of ascarid impaction,51 and epiploic foramen entrapments are identified as edematous loops of small intestine found in the right cranial abdomen.54 The veterinarian may note small intestinal intussusceptions as targetlike lesions when viewed in cross sections.55 The presence of bowel loops, stomach, or liver in the thoracic cavity indicates the presence of herniation through the diaphragm and should be confirmed using radiography or surgical exploration.
Evaluation of the large intestine may be difficult because of the large amounts of gas within the lumen. It is also extremely difficult to reliably differentiate between specific segments of the colon ultrasonographically.56 However, certain disorders are readily identifiable through ultrasonography. The nephrosplenic ligament area can be assessed for bowel entrapment in the left paralumbar fossa. In cases of entrapment the spleen will be pulled away from the body wall, and fluid or gas shadows will be observable dorsal to the spleen, obscuring the kidney, which is normally adjacent and abaxial to the spleen.57 Small colon, small intestine, or pneumoperitoneum also may produce a gas shadow and obscure the kidney from view.51
Sand impactions may appear as hyperechoic bands on the ventral abdominal wall,51 but ultrasound does not describe the extent of sand accumulation as well as radiographic assessment.58 Ileocecal and cecocolic intussusceptions may be visible in the upper right paralumbar fossa.59 In cases of colitis large, fluid-filled colons may be visible with or without intramural edema. The right dorsal colon is consistently located abaxial to the liver, within the right thirteenth to fifteenth intercostal space, and may be thickened (>5 mm) in cases of right dorsal colitis. Ultrasound also can be a useful tool for evaluating changes in motility over time in a given location.60
Nuclear Scintigraphy
The procedure requires special gamma cameras and the injection of radioactive materials into the bloodstream. One of two methods may be used: injection of technetium-99m methylene diphosphonate (99mTc-MDP) directly into the blood or injection of 99mTc-labeled leukocytes.61 The principle of nuclear scintigraphy then lies in increased uptake of the dye or the white blood cells into areas of inflammation. One of the most common uses of nuclear scintigraphy in evaluating the gastrointestinal tract is diagnosis of dental disease. Scintigraphy using 99mTc-MDP proved to be more sensitive in cases of dental disease than was radiography. Scintigraphy was slightly less specific, however, and therefore should be used with radiography or computed tomography for ultimate accuracy.62 Scintigraphy using radiolabeled white blood cells can support a diagnosis of right dorsal colitis in the horse.63 Images taken of the abdomen 20 hours after injection showed an increased linear uptake of leukocytes in the region of the right dorsal colon in horses with right dorsal colitis compared with normal horses. Other uses of nuclear scintigraphy include evaluation of metastasis of abdominal tumors to bony areas, assessment of biliary kinetics, and determination of liquid- and solid-phase gastric emptying.62–64
Advanced Imaging
Computed tomography (CT) is becoming increasingly available, and as such, various references are currently available describing the normal anatomy of the equine head as imaged with this modality.65,66 CT is extremely useful to evaluate dental disease as well as tumors and masses of the head, larynx, pharynx, and proximal esophagus.67,68 CT also has promise for evaluating abdominal disorders in foals. Most equipment can accommodate animals up to 400 lb. Restrictions of CT as a diagnostic aid include expense, availability, and weight and size limitation.
Tests of Absorption and Digestion
d-Glucose or d-xylose absorption tests are useful in determining malabsorption of carbohydrates from the small intestine in horses. The protocol for absorption tests using either carbohydrate is similar. The horse should be fasted for 18 to 24 hours before testing. Increased periods of fasting actually have been shown to decrease absorption of d-xylose and interfere with results.69 A dosage of 0.5 to 1 g/kg of d-glucose or d-xylose is administered through a nasogastric tube. Administration of sedatives may falsely increase the blood glucose levels and interfere with gastrointestinal transit times. Blood samples are collected to measure glucose or xylose concentrations at 0, 30, 60, 90, 120, 150, 180, 210, and 240 minutes after administration. Additional samples can be taken up to 6 hours after dosing if the results are questionable. One should measure glucose in blood samples collected with sodium fluoride as an anticoagulant and measure xylose in samples collected in heparinized plasma.
A normal d-glucose absorption test, also known as an oral glucose tolerance test, should have a peak between 90 and 120 minutes, and this peak should be greater than 85% above the resting glucose value.70 Complete malabsorption is defined as a peak less than 15% above the resting levels, and partial malabsorption is defined as a peak between 15% and 85% above the resting level. It is important to remember that gastric emptying, gastrointestinal transit time, length of fasting, cellular uptake and metabolism, age, diet, and endocrine function influence glucose absorption curves.70,71 Malabsorption demonstrated by the oral glucose tolerance test is sensitive but not specific. Diseases that may cause a lowered or delayed peak include infiltrative lymphosarcoma, inflammatory bowel disease (lymphocytic-plasmacytic or eosinophilic), cyathostomiasis, chronic colitis (Salmonella sp.), multisystemic eosinophilic epitheliotropic disease, food allergies, and small intestinal bacterial overgrowth.72 d-Xylose absorption tests have some advantages over the oral glucose tolerance test because xylose is not metabolized in the small intestinal mucosa and insulin does not influence its absorption. Gastric and intestinal motility, intraluminal bacterial overgrowth, and renal function still influence xylose absorption because the kidneys clear xylose.72 The other main drawback to d-xylose is that it is generally available only in research settings. However, xylose measurements are available at many major universities. A normal d-xylose absorption curve should peak between 20 and 25 mg/dl at 60 to 120 minutes after dosing.73 Decreased xylose absorption can occur in horses with inflammatory bowel disease, lymphosarcoma, multisystemic eosinophilic epitheliotropic disease, cyathostomiasis, extensive small intestinal resections, and any cause of villous atrophy.72
Maldigestion is a common occurrence in foals with diarrhea. Bacteria (especially Clostridium sp.) and viruses (especially rotavirus or coronavirus) may invade and destroy the villous epithelial cells that manufacture lactase and other disaccharidases, resulting in an inability to digest lactose. In this case continued ingestion of the mare’s milk may cause an osmotic diarrhea, which may exacerbate the underlying enterocolitis. The veterinarian can perform lactose tolerance testing to assess the degree of maldigestion by administering d-lactose at 1 g/kg as a 20% solution via nasogastric tube and measure glucose concentrations in the blood at 0, 30, 60, 90, 120, 150, 180, 210, and 240 minutes. A normal curve shows doubling of glucose levels compared with baseline by 60 minutes after administration.74
Evaluation of Gastric Emptying
Multiple diagnostic imaging techniques have been used to study gastric emptying times. Contrast radiography can be used to assess gastric emptying in foals. In the normal foal barium remains in the stomach for varying amounts of time, but a significant amount should be gone within 2 hours.48 Gastric emptying of solid, nondigestible, radiopaque markers also has been used in adult horses and ponies, but the results were variable and unpredictable even in the normal horse.75 Nuclear scintigraphy is used commonly in human beings to measure gastric emptying and can be used in horses when available. The technique for measurement of liquid gastric emptying requires oral administration of 99mTc pentenate (10 mCi) and serial images taken of the cranial abdomen. The tracer is usually not visible 1 hour after administration in normal horses.64 An adaptation of this methodology can be used for measurement of solid phase emptying of a 99m Tc–labeled pelleted ration.76
Alternatively, if nuclear scintigraphy is not available, acetaminophen absorption testing can be used as an indirect determination of liquid gastric emptying.77,78 This test is performed by administering 20 mg/kg of acetaminophen orally and measuring subsequent blood values, then calculating the time to reach maximum serum concentrations and the absorption constant. In human beings the proximal small intestine absorbs almost all of the acetaminophen.79 The median time to reach peak plasma levels using acetaminophen absorption in horses was 47.7 minutes.77
The 13C-octane acid breath test offers an easy, noninvasive method of determining gastric emptying of solids.76,80 This test is performed by feeding a standard 13C-labeled test meal, and then collecting breath samples using a modified mask. The breath is then analyzed for the ratio of the novel isotope, 13:CO2, to the normally produced 12:CO2.
HISTOPATHOLOGIC EXAMINATION
It is often necessary to perform a histopathologic examination of tissues from the intestine to diagnose chronic inflammatory, infiltrative, or neoplastic conditions, and such examination can be useful in evaluating the extent of injury after obstruction or ischemia. Rectal mucosal biopsies are easy to collect, with few complications. However, to collect a full-thickness biopsy of the intestine requires a surgical approach (flank or ventral midline approach). Laparoscopy offers a safer technique to observe the large intestine and other abdominal structures.81 The practitioner can obtain biopsies of masses, lymph nodes, and mesentery or intestinal serosa using laparoscopy and mucosal biopsies of the upper gastrointestinal tract using endoscopy.
LAPAROSCOPY
Other diagnostic tools, specifically laparoscopy and CT, are available but require specialized equipment and personnel with specific training. Flexible or rigid endoscopes used for laparoscopic evaluation of the abdomen allow for visualization of visceral organs and potentially for collection of biopsy material from masses or organs. Full-thickness biopsies of the intestines are not routinely possible through the laparoscope and usually require flank or ventral midline laparotomy. The laparoscopic procedure can be done with the horse standing or recumbent. Advantages of this technique over a flank or ventral midline celiotomy include smaller incisions, shorter healing time, and shorter procedure time. Disadvantages include the large amount of equipment needed, the high degree of skill involved, and the limitation as a diagnostic modality, rather than a treatment.82 Clinical applications of diagnostic laparoscopy include the correction of rectal tears; percutaneous abscess drainage, assessment of adhesions, displacements, and integrity of the serosa of various bowel segments; and biopsy of abdominal masses.81
INITIATION OF THE INFLAMMATORY RESPONSE
Epithelium
The gastrointestinal epithelium interfaces with a luminal environment that is inhabited by potentially hostile microbial organisms. The epithelium presents a physical barrier to invasion by the flora of the gastrointestinal tract, consisting of the apical cellular membrane, intercellular tight junctions (the permeability of which is highly regulated), and a secreted layer of mucus. When invading pathogens breach the mucosal barrier, potent soluble and neural signals are generated that initiate an inflammatory response.1 The epithelium can be conceptualized as a sensory organ that detects pathogen invasion to trigger an appropriate host defense and reparative response.
Noninfectious mucosal injury or invasion of epithelial cells by pathogenic organisms such as Salmonella activates the synthesis of pro-inflammatory chemokines (chemoattractants) by epithelial cells that triggers a robust influx of neutrophils into the tissue within hours of the damage. 1 Of the chemoattractants produced by epithelium, interleukin-8 (IL-8) has a particularly important role in initiating inflammation by recruiting neutrophils from blood2–4 and regulating neutrophil migration through tissue matrix adjacent to epithelium.5,6 Complement fragments such as C5a and bacteria-derived formylated chemotactic peptides also act as potent “end target” chemoattractants that are fully capable of stimulating a robust inflammatory response in the intestine if the epithelial barrier permits invasion of bacteria or the diffusion of bacterial peptides across the mucosa.
Epithelial cells activated during infection produce cytokines such as tumor necrosis factor α (TNF-α), arachidonic acid metabolites, and other pro-inflammatory mediators that activate recruited leukocytes.7 Microbial products, particularly lipopolysaccharide and other bacterial cell wall components and microbial nucleic acids, are potent activators of leukocytes recruited into the tissue.8 Mast cells are key sentinel leukocytes that sense microbial invasion, releasing TNF-α that appears to be a critical initiator and regulator of the cellular phase of inflammation.9 Once the inflammatory response has been initiated, TNF-α; IL-1β; and other pro-inflammatory products of neutrophils, monocytes, mast cells, and epithelial cells amplify the inflammatory response.
The enteric nervous system has a key role in sensing and regulating inflammatory responses in the intestine. For example, Clostridium difficile toxin A activates a neural pathway that triggers mast cell degranulation and neutrophil influx into the tissue.10,11 Blockade of this neural pathway is sufficient to abolish the profound inflammatory response induced by toxin A as well as many of the effects of toxin A on enterocyte secretion. Other pathogens and immune-mediated hypersensitivity reactions similarly stimulate inflammation by mechanisms that involve the enteric nervous system. Thus the epithelium interacts in a highly complex manner with the intestinal milieu, the enteric nervous system, and inflammatory cells to regulate the tissue response to injury and infection.
Macrophages
Resident macrophages located in the lamina propria, submucosa, and intestinal lymphoid organs are among the first cells beyond the epithelium to respond to infection or injury. Macrophages are activated by microbial products by way of pattern recognition receptors and begin to produce pro-inflammatory molecules important for recruiting and activating neutrophils and monocytes. Pattern recognition receptors recognize microbial molecules such as lipopolysaccharide, lipoproteins, flagellin, peptidoglycan, and nucleic acids to signal the invasion by pathogens.8 Of the pattern recognition receptors, the lipopolysaccharide (LPS) receptor complex is perhaps the best defined. LPS activates macrophages by way of the CD14-Toll-like receptor 4 complex to initiate transcription of the inflammatory cytokines TNF-α and IL-1β, which synergize with LPS to amplify the macrophage response.8 LPS, particularly in concert with inflammatory cytokines, stimulates macrophages to produce copious amounts of nitric oxide, which is both microbicidal and vasoactive.12 Nitric oxide and other nitrogen radicals react with reactive oxygen intermediates (ROIs) generated by the activated oxidase complex to produce some of the most toxic molecules of the host defense system: the peroxynitrites.12 IL-8 is produced as well to recruit neutrophils. As the response progresses, other inflammatory mediators, particularly the arachidonic acid–derived lipids dependent on inflammation-induced cyclooxygenase-2 and 5-lipoxygenase activity, are produced that have potent vasoactive and pro-inflammatory effects through the activation of endothelial cells, neutrophils, and platelets13.
VASCULAR RESPONSE DURING INFLAMMATION
Four important changes occur in the intestinal vasculature during inflammation: (1) alteration of blood flow; (2) increased vascular permeability; (3) increased adhesiveness of endothelial cells, leukocytes, and platelets; and (4) exposure of the basement membrane and activation of the complement, contact, and coagulation cascades.
Increased vascular permeability is initially caused by inflammatory mediator actions on the endothelial cells. Histamine, leukotrienes, PAF, prostaglandins, bradykinin, and other mediators stimulate endothelial cell contraction, and interendothelial gaps form.14,15 This stage of increased vascular permeability is readily reversible. Concurrently, mediators such as the cytokines TNF-α and IL-1β induce a structural reorganization of the interendothelial junctions, resulting in frank discontinuities in the endothelial monolayer.16 Cytokines also stimulate endothelial cells to express adhesion molecules that support adhesion of leukocytes and platelets,17 leading to the next and perhaps most devastating event. Leukocytes (primarily neutrophils) and platelets adhere to exposed basement membranes and activated endothelial cells. Adherent neutrophils and platelets are then exposed to the mediators of inflammation present in the surrounding milieu, which activates the cells to release oxidants and proteases (particularly elastase) that injure the endothelium and have the potential to cause irreparable harm to the microvasculature.18–20 Marginated neutrophils begin to transmigrate between endothelial cells (as described in later sections), which, if in sufficiently large numbers, disrupt the integrity of the interendothelial junctions, worsening the vascular leakage.19
These stages of enhanced vascular permeability can be conceptualized as a mechanism to allow plasma proteins to enter the tissues and to potentiate the critical influx of leukocytes into tissues. However, if they are not regulated precisely, alterations in both hydrostatic and oncotic forces and irreversible damage to the vascular bed may have devastating consequences. Moreover, inappropriate activation of plasma protein cascades and leukocytes by activated endothelium and exposed matrix proteins can contribute to systemic inflammation (systemic inflammatory response syndrome, or SIRS; see the section on gastrointestinal ileus for more information) characterized by hypotension, generalized vascular leak syndrome, and multiorgan dysfunction, which may be fatal. Phosphodiesterase inhibitors reduce endothelial permeability in ischemia-reperfusion injury and other models of inflammation-induced vascular leakage21,22 by increasing endothelial tight junction integrity and thus may be a viable therapeutic strategy to prevent or reduce the permeability alterations associated with inflammation.
CELLULAR EFFECTORS OF INFLAMMATION
Neutrophils
RECRUITMENT
Infection or injury to the gastrointestinal mucosa causes an influx of leukocytes from the blood that lay the foundation of the inflammatory response. Neutrophils, the first to arrive during inflammation, have a dominant role in the acute response. Within minutes neutrophils are recruited into the tissue, where they are activated to release products that not only are lethal to pathogens and pro-inflammatory but also may damage host cells and tissues.23 Not surprisingly, much attention has been paid to the role of neutrophils in the pathophysiology of many inflammatory conditions.24 Neutrophil depletion is protective in many models of gastrointestinal inflammatory disease. Of interest to clinicians, blockade of neutrophil migration into inflamed tissues prevents many of the pathophysiologic events associated with infectious enteritis, ischemia-reperfusion injury, and other gastrointestinal diseases.18,25–29
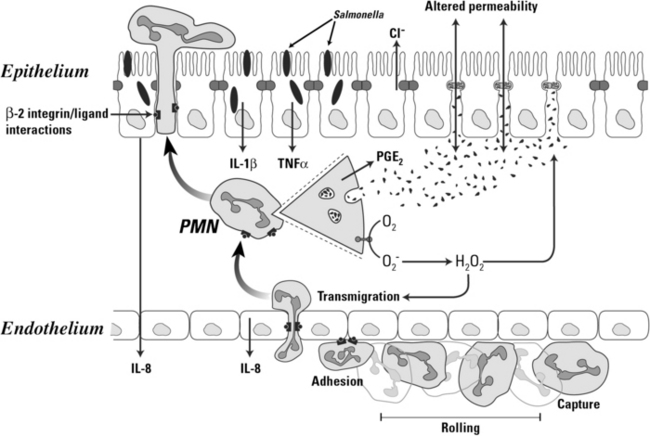
Neutrophil transendothelial migration is a multistep process that is temporally and spatially regulated and has a degree of cell type specificity (Figure 15-2). The predominant sites of neutrophil transendothelial migration are in the postcapillary venules and, in some tissues, capillaries. Endothelial cells in these vessels respond to cytokines and other soluble signals by expressing molecules that promote neutrophil adhesion and transmigration, including selectins and counter receptors for integrins. As neutrophils flow through these vessels, they are first tethered to activated endothelium. Tethering is mediated by selectin molecules expressed on neutrophils (L-selectin) and on activated endothelial cells (P- and E-selectins), which bind to PSGL-1, ESL-1, and other mucin counter-receptors.30,31 The function of tethering is to increase the exposure of the neutrophil to activating chemokines presented on the surface of the endothelial cells.
Stimulation of neutrophils by IL-8 and other chemokines activate the second step of transendothelial migration. Chemokine binding to their receptors on the neutrophil generates signals that activate the binding of integrin adhesion receptors to their ligands, called intracellular adhesion molecules (ICAMs) or vascular cell adhesion molecules (VCAMs), expressed on endothelial cells in inflamed mucosa. Integrin ligation to ICAMs arrests the tethered neutrophils, resulting in firm adhesion to the endothelium. Of the integrins expressed on neutrophils, the β2 integrins have a particularly important role in transendothelial migration. Calves and people with the disorder leukocyte adhesion deficiency (LAD) illustrate the requirement for β2 integrin-mediated adhesion in neutrophil function. LAD is a result of an autosomal recessive trait resulting in the lack of the β2 integrin expression. The neutrophils from affected individuals cannot migrate into most tissues and do not function normally, resulting in poor tissue healing and profound susceptibility to infection, especially at epithelial barriers.32,33 Other integrins also have a role in transendothelial migration. β1 Integrins mediate transendothelial migration in some cells and seem to be particularly important for mediating emigration of monocytes into many tissues.34
Following this firm adhesion step, neutrophils migrate through the endothelium along a chemotactic gradient of IL-8 and other chemoattractants, such as C5a and LTB4.3,19,35 Neutrophils migrate across the endothelial monolayer at intercellular junctions by way of a mechanism involving a series of integrin-ligand interactions mediated by both β2 and β1 integrins and other adhesion molecules31 that is generally capable of maintaining the integrity of the endothelial barrier.36 However, massive flux of neutrophils through the endothelium alter endothelial tight junctions and injure the basement membrane, resulting in increased endothelial permeability to molecules as large as plasma proteins and even endothelial cell detachment from the basement membrane.19,20 Nonintegrin molecules such as platelet-endothelial cell adhesion molecules (PECAMa) also are involved in transendothelial migration of neutrophils.31 Homotypic binding of PECAMs on adjacent endothelial cells form part of the intercellular junction. Neutrophils express an integrin of the β3 family that can bind PECAM, and through sequential binding of β3 integrins to PECAM, the neutrophil can “unzip” the intercellular junction and migrate through, closing it behind itself.
ACTIVATION
A key feature of neutrophils and other leukocytes is the requirement for integrin-mediated adhesion to extracellular matrix (ECM) proteins or other cells to achieve an optimal effector phenotype.37 Critical components of the ECM in inflamed tissues include fibronectin, fibrinogen, and vitronectin, deposited in tissues as a result of plasma leakage and by synthesis of new proteins by stromal cells and resident macrophages in response to inflammatory mediator activation. The changing composition of the matrix proteins deposited in tissues during inflammation serves as a cue as to the nature of the tissue environment for recruited inflammatory cells as they become activated. Individual gene expression studies have demonstrated that adhesion to matrix proteins induces the expression of cytokines and chemokines and their receptors, arachidonic acid–derived lipid mediator synthases, metalloproteinases, growth factors, transcription factors, and other genes that influence the differentiation and activation of inflammatory cells.38
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree