CHAPTER 21 Disorders of Foals
NEONATAL AND PERINATAL DISEASES
Before the 1980s intensive management of the compromised neonate was unusual and little was known regarding many of the problems of this special patient population. Although some specific conditions had been described by astute clinician-researchers, most notably the “dummy” foal syndrome1 and respiratory distress syndrome caused by primary surfactant deficiency,2 little information regarding the diagnosis and management of conditions of the foal during the neonatal period was available, although at least one active group was investigating fetal and neonatal physiology of the horse in Great Britain.1–14 When treatment of compromised foals was undertaken, the approach usually resembled treating them as small adults, with little consideration given to the different physiology of the equine neonate. The advent of improved management of reproductive efficiency in mares led naturally to an increased interest in preservation of the conceptus to parturition and the foal thereafter. Interested clinicians, drawing on lessons from human perinatology and neonatology and sometimes collaborating with their counterparts in the human field, pioneered investigations of these small patients and created the fields of equine perinatology and equine neonatal intensive care.1–19 Because of the foresight and energy of these early investigators, the field of veterinary perinatology-neonatology exploded in the 1980s, leading to the creation of equine neonatal intensive care units throughout the United States and the world. From these units information about the normal and abnormal physiology of foals, the medical conditions affecting them, and methods for treatment and management of these problems has been developed through observational, retrospective, and prospective studies. This veritable explosion of information over the last 20 years has greatly improved the ability of all practitioners to provide appropriate care for these patients, whether in the field or at an equine neonatal intensive care unit. The ability not only to save the lives of these patients but also to treat them in such a manner as to allow them to fulfill their purposes, whether as pleasure animals or racing athletes, has improved almost exponentially from those early days.20–23 This section aims to provide the clinician with current information about the management of these patients, with the recognition that much still remains unknown and that advances will continue to be made in this dynamic field. The reader is cautioned that much of this chapter is flavored by the experiences of the authors and that variation in the approach to and treatment of specific problems exists among neonatal intensive care units (NICUs) and among clinicians in the same NICU; moreover, each year results in change. In some cases information that is presented has been gleaned from human NICU studies, essentially using the critically ill infant as the experimental model.
Many of the problems of the newborn foal have their genesis in utero. Identification of high-risk pregnancies is an important component of prenatal care of the foal, and some of the most commonly encountered problems of the dam that result in abnormal foals include previous or concurrent disease, poor reproductive history, poor perineal or pelvic conformation, poor general health, poor nutritional condition, prolonged transport, history of previous abnormal foals, placental abnormalities, and twins.24
Some of the more common causes of abortion can result in the birth of severely compromised foals of variable gestation lengths (Box 21-1). These include infectious causes such as equine herpesvirus (EHV) types 1 (most commonly) and 4 (rarely), equine infectious anemia, equine arteritis virus, bacterial and fungal placentitis, leptospirosis, equine ehrlichiosis, and septicemia-endotoxemia.25–27 Noninfectious causes of abortion include twinning and noninfectious placental abnormalities such as extensive endometrial fibrosis, body pregnancy, and abnormal length (long or short) of the umbilical cord.24,28
PREPARTUM EVALUATION OF THE FETUS AND PLACENTA
Once a pregnancy has been established as high risk, the fetus should be evaluated for viability. Evaluation should include as thorough an evaluation as possible of the reproductive tract, placenta, and fetal fluids. The details of this examination are described in Chapter 18. Prepartum disorders in the mare usually are readily recognizable, but disorders of the fetus and placenta can be more subtle and difficult to determine. The first step is to take a thorough history of the mare. Of particular interest is any history of previous abnormal foals, but the history taking should include questions regarding transportation; establishment of an accurate breeding date (sometimes more difficult than one would suspect); pertinent medical history, including any diagnostic testing performed for this pregnancy, such as culture, endometrial biopsy, and cytologic results; and any rectal and ultrasound examination results. Additionally, the clinician should obtain information regarding the possible ingestion of endophyte-infected fescue or exposure to potential infectious causes of abortion.29,30 A complete vaccination and deworming history is requisite, as is a complete history of any medications and supplements administered during pregnancy.
After obtaining a history, the clinician performs a rectal examination, including palpation of the cervix, uterus, fetus, and all palpable abdominal contents. Any abnormalities should be noted. The cervix should be tight throughout gestation; the late-gestation uterus will be large and distended with fluid and usually pulled craniad in the abdomen. Palpation of the fetus frequently results in some fetal movement; however, lack of movement should be interpreted with caution insofar as some normal fetuses do not respond. Ultrasonographic evaluation of the uterus and conceptus by way of the rectum can provide valuable information, particularly regarding placental thickness if placentitis is a concern. The clinician may evaluate fetal fluids and estimate fetal size on the basis of the size of the eye later in gestation.31 In some hospitals the practitioners choose not to perform vaginal or speculum examinations because of an association between these examinations and the subsequent development of placentitis. Unless placentitis is recognized with ultrasonographic evaluation by way of the rectum and culture is desirable, these types of examinations generally are not necessary.
After rectal examination a transabdominal ultrasonographic evaluation of the uterus and conceptus is performed.28 The neonatologist can generate a biophysical profile of the fetus on the basis of this examination in the late-term fetus and readily determine viability.32,33 It is also possible to determine the presence or absence of twins in the late pregnant mare in this manner. The sonogram is performed through the acoustic window from the udder to the xiphoid ventrally and laterally to the skin folds of the flank. Imaging of the fetus usually requires a low-frequency (3.5-MHz) probe, whereas examination of the placenta and endometrium requires a higher-frequency (7.5-MHz) probe. A complete description of this examination is beyond the scope of this chapter, but several complete descriptions of the technique and normal values for specific gestation lengths can be found by consulting the relevant veterinary literature.33 The utility of this examination lies in its repeatability and low risk to the dam and fetus. Sequential examinations over time allow the clinician to follow the pregnancy and identify changes as they occur.
A companion to transabdominal ultrasonography is evaluation of the fetal electrocardiogram (ECG). Fetal ECGs can be measured continuously using telemetry or obtained through more conventional techniques several times throughout the day.24,28,34 The operator places electrodes on the mare’s skin in locations aimed at maximizing the magnitude of the fetal ECG. Because the fetus frequently changes position, multiple sites may be needed in any 24-hour period. To begin, one places an electrode dorsally in the area of the sacral prominence, with two electrodes placed bilaterally in a transverse plane in the region of the flank. The fetal ECG maximal amplitude is low, usually 0.05 to 0.1 mV, and can be lost in artifact or background noise, so it is often necessary to move electrodes to new positions to maximize the appearance of the fetal ECG. The normal fetal heart rate during the last months of gestation ranges between 65 to 115 beats/min, a fairly wide distribution. The range of heart rate of an individual fetus can be narrow, however. Bradycardia in the fetus is an adaptation to in utero stress, most commonly thought to be hypoxia. By slowing the heart rate, the fetus prolongs exposure of fetal blood to maternal blood, increasing the time for equilibration of dissolved gas across the placenta and improving the oxygen content of the fetal blood. The fetus also has altered the distribution of its cardiac output in response to hypoxia, centralizing blood distribution.35,36 Tachycardia in the fetus can be associated with fetal movement, and brief periods of tachycardia should occur in the fetus in any 24-hour period. Persistent tachycardia is a sign of fetal distress and represents more severe fetal compromise than bradycardia. The author has recognized dysrhythmias in the challenged fetus, most commonly as atrial fibrillation but also as apparent runs of ventricular tachycardia.
The ability to monitor the fetus in a high-risk pregnancy inevitably has led to questions of whether, how, and when to intervene. Most equine neonatologists would agree that removal of the fetus from the uterus before it is ready for birth is not desirable. One of the difficulties in determining fetal preparedness for birth is that prediction of parturition is difficult in these mares. Many of the parameters used in normal mares are unreliable in the high-risk pregnant mare. One must have an accurate history of any previous gestation length in terms of days for the mare in question to allow a more accurate estimate of her usual gestational length. Evaluation of the usual mammary gland parameters, including size, the presence of “wax,” and alteration of electrolyte concentrations, is not generally predictive in the high-risk mare; in the author’s experience many of these mares have changes predictive of parturition for weeks before actual parturition.37,38 This circumstance may be related to the observation that many high-risk pregnant mares, particularly those with placentitis, are presented for a primary complaint of early onset lactation. Although pulmonary system maturity in human beings can be assessed with some degree of accuracy using measurement of lecithin-sphingomyelin ratios, this measurement—along with sphingomyelin, cortisol, and creatinine concentrations in the amniotic fluid—has proved to be of no benefit in the horse.39–41 Amniocentesis carries a high risk of abortion in the horse, even with ultrasound guidance, and is not a clinically useful technique at this time.41 Currently, no clear-cut guidelines are available indicating the best time to intervene, but the presence of persistent fetal tachycardia or prolonged absence of fetal movements, including breathing movements, as determined by transabdominal ultrasound evaluation, should initiate discussion regarding the appropriateness of induction of parturition or elective cesarean section. The goal of induction or cesarean section is to remove a pregnancy that is threatening the survival of the dam, with no thought to fetal survival, or to remove the fetus from a threatening environment to improve its likelihood to survive. Preterm induction is ill advised if fetal survival is desirable because of the limited ability to treat severely immature neonates. Timing of intervention in these circumstances remains an art, not a science.
The approach to management of the high-risk pregnancy is dictated to some degree by the exact cause for concern, but for many mares therapy is similar. Many high-risk mares have placentitis, primarily caused by ascending bacterial or fungal infections originating in the region of the cervix. These infections can cause in utero sepsis or compromise the fetus by local elucidation of inflammatory mediators or altered placental function.42,43 Premature udder development and vaginal discharge are common clinical signs. Treatment consists of administration of broad-spectrum antimicrobial agents and nonsteroidal anti-inflammatory drugs (Table 21-1). In some clinics trimethoprim-sulfonamide drugs have been the antimicrobial of choice because of unpublished studies that demonstrated an increased concentration of these agents in the fetal fluids compared with penicillin and gentamicin. However, if culture and sensitivity results are available, directed therapy should be instituted. Nonsteroidal anti-inflammatory agents such as flunixin meglumine are useful to combat alterations in prostaglandin balance that may be associated with infection and inflammation. Although the efficacy of these agents is best when administered before the development of clinical signs, to date no detrimental effects have been reported in the fetus or dam when chronically used at low doses in well-hydrated patients. Tocolytic agents and agents that promote uterine quiescence include altrenogest, isoxuprine, and clenbuterol.44–48 Usually, altrenogest is administered, although its need in late gestation has been challenged. The efficacy of isoxuprine as a tocolytic in the horse is unproven, and the bioavailability of orally administered isoxuprine appears to be highly variable.48 The long-term use of clenbuterol is inadvisable because of receptor population changes associated with chronic use and its unknown effects on the fetus at this time. Clenbuterol may be indicated during management of dystocia in preparation for assisted delivery or cesarean section.46 The intravenous form of clenbuterol currently is not available in the United States.
TABLE 21-1 Drugs Used to Treat High-Risk Pregnancy
| Drug | Dose/Frequency/Route | Reason |
|---|---|---|
| Trimethoprimsulfonamide | 25 mg/b.i.d.; PO | Antimicrobial |
| Flunixin meglumine | 0.25 mg/kg t.i.d.; PO or IV | Anti-inflammatory |
| Altrenogest | 0.44 mg/kg s.i.d.; PO | Tocolytic |
| Isoxuprine | 0.4-0.6 mg/kg b.i.d.; IM∗ or PO | Tocolytic |
| Clenbuterol | 0.8 μg/kg as needed PO∗ | Tocolytic |
| Vitamin E | 6000-10,000 IU s.i.d.; PO | Antioxidant |
b.i.d., Twice daily; t.i.d., three times daily; PO, per os (by mouth); IV, intravenous; s.i.d., once daily; IM, intramuscular
∗ Intravenous form currently not available in the United States.
Three additional strategies can be used in managing mares with high-risk pregnancies. In mares with evidence of placental dysfunction, with or without signs of fetal distress, the author provides intranasal oxygen supplementation in the hope of improving oxygen delivery to the fetus. Intranasal oxygen insufflation of 10 to 15 L/min to the mare significantly increases partial pressure of oxygen (Pao2) and percent oxygen saturation of hemoglobin.49,50 Because of the horse’s placental vessel arrangement, improvement of these two arterial blood gas parameters should result in improved oxygen delivery to the fetus. Blood gas transport is largely independent of diffusion distance in the equine placenta, particularly in late gestation, and depends more on blood flow. Information from other species cannot be extrapolated to the equine placenta because of its diffuse epitheliochorial nature and the arrangement of the maternal and fetal blood vessels within the microcotyledons.51,52 Umbilical venous pO2 is 50 to 54 mm Hg in the horse fetus, compared with 30 to 34 mm Hg in the sheep, whereas the maternal uterine vein to umbilical vein pO2 difference is near 0. Also unlike the sheep, the umbilical venous pO2 values decrease 5 to 10 mm Hg in response to maternal hypoxemia and increase in response to maternal hyperoxia.53–55
Vitamin E (tocopherol) is administered orally to some high-risk mares as an antioxidant. Administration of large doses of vitamin E before traumatic brain injury improves neurologic outcome in experimental models and has been examined as possible prophylaxis for human neonatal encephalopathy.56–58 Extrapolation of that information to the compromised equine fetus suggests that increased antioxidant concentrations in the fetus may mitigate some of the consequences of uterine and birth hypoxia, but no evidence is available to date demonstrating that protection occurs or that vitamin E accumulates in the fetus in response to supplementation of the mare. Finally, many high-risk mares are anorectic or restricted from feed because of their medical condition. These mares are at particularly great risk for fetal loss because of their lack of feed intake, which alters prostaglandin metabolism.59 Therefore the clinician should administer 2.5% to 5% dextrose in 0.45% saline or water (5% dextrose) intravenously at maintenance fluid rates to these patients.
Perhaps the most important aspect of managing mares with high-risk pregnancies is frequent observation and development of a plan. These mares should be observed at least hourly for evidence of early-stage labor and should be under constant video surveillance if possible. Depending on the primary problem, the team managing the mare should develop a plan for handling the parturition once labor begins and for fetal resuscitation after delivery. Any equipment that may be needed should be readily available stallside, and a call sheet listing contact numbers for all involved should be posted on or near the stall. The plan should include a description of how a complicated dystocia would be handled, should it occur, with permission for general anesthesia and cesarean section obtained before the event so that time is not wasted. An important question to be posed to the owner at the outset is whether the mare or the foal is more important; this answer may dictate the direction of the decision tree once labor begins.60,61
EVALUATION OF THE NEWBORN FOAL
Early recognition of abnormalities is of utmost importance for the successful management of critically ill foals. To recognize the abnormal, the normal must be known. Immediately after birth, several important physiologic and behavioral changes occur. Chief among these changes are the adaptation of the cardiovascular and respiratory systems to extrauterine life; thus persistent increases or decreases in heart or respiratory rates should alert the clinician to existing or impending problems. The normal transition of the respiratory tract involves opening closed alveoli and absorption of fluid from the airway, accomplished by a combination of breathing efforts, expiration against a closed glottis (“grunting”), and a change in sodium flux across the respiratory membrane from net secretion to net absorption.1–5 The transition from fetal to neonatal circulatory patterns requires resolution of the pulmonary hypertension present in the fetus, normally shunting blood flow through the lower resistance ductus arteriosus in the fetal state, in order to direct cardiac output to the pulmonary vasculature for participation in gas exchange. This change is achieved by opening alveoli; decreasing airway resistance and providing radial support for pulmonary vessels; achieving functional closure of the ductus arteriosus; and increasing the oxygen tension in the lung, reversing pulmonary vasoconstriction mediated by hypoxia.6,7 Pulmonary tree vasodilators (prostacyclin, nitric oxide [NO]) and vasoconstrictors (endothelin-1, leukotrienes) play apparently well-coordinated, but as yet not fully elucidated, roles. In the normal newborn this change is smooth and rapid. These critical events are undermined by factors such as inadequate lung development, surfactant deficiency (primary or secondary), viral or bacterial infection, placental abnormalities, in utero hypoxia, and meconium aspiration.
Spontaneous breathing should begin in the neonate within 1 minute of birth; many foals will attempt to breathe as their thorax clears the pelvic canal.8,9 First breaths are normally triggered by the combination of removal of placental humoral inhibitory factor, cooling of the neonate, tactile stimulation, the catecholamine surge’s induction of substances important for breathing (e.g., substance P) and increasing carbon dioxide. Apnea at birth can be caused by asphyxia, central nervous system (CNS) depression from maternal drugs, CNS injury, septicemia, anemia, primary muscular or neurologic disease, and obstructing congenital malformations or other mechanical obstruction of the airway. During the first hour of life, the respiratory rate of a healthy foal can be as high as 80 breaths per minute but should decrease to 30 to 40 breaths per minute within a few hours. Similarly, the heart rate of a healthy newborn foal will have a regular rhythm and be at least 60 beats per minute at the first minute.8,9 By the time the foal is 1 day of age, the heart rate should range between 70 to 100 beats per minute. Persistent bradycardia can be caused by hypoxic damage, acid-base derangements, and sepsis and is an indication for rapid intervention. A continuous murmur can usually be ausculted over the left side of the heart, although its loudness may vary with position. This murmur is thought to be associated with some shunting through the ductus arteriosus. Variable systolic murmurs, thought to be flow murmurs, may be ausculted during the first week of life.10 Murmurs that persist beyond the first week of life in an otherwise healthy foal should be more thoroughly investigated, as should any murmur associated with persistent hypoxia. Sinus arrhythmias can be noted relatively frequently during the immediate postpartum period. Various other arrhythmias (e.g., ventricular premature complexes, ventricular tachycardia, supraventricular tachycardia) occasionally can be observed in normal foals; however, all arrhythmias should disappear within 15 minutes post partum.11 Physiologic changes associated with the adaptive period are probably the main factors contributing to these arrhythmias. The clinical significance of any unusual or abnormal arrhythmias should take into consideration clinical, metabolic, and homodynamic findings of the neonate.
Auscultation of the thorax shortly after birth will reveal a cacophony of sounds as airways are opened and fluid is cleared. End-expiratory crackles are consistently heard in the dependent lung during and after lateral recumbency. Changes in respiratory rate, effort, or breathing pattern are more appreciable than auscultable changes in neonates with respiratory disease. Premature and dysmature foals or foals subjected to peripartum hypoxia can demonstrate abnormal breathing patterns, especially when sleeping. Any sudden change in respiratory parameters should be investigated immediately because it may indicate deterioration in the foal’s condition. It is not unusual for a normal newborn foal to appear slightly cyanotic during this initial adaptation period, but this should resolve within minutes of birth. The equine fetus, like all fetuses, exists in a moderately hypoxic environment, but the equine fetus has a greater partial pressure of oxygen, approximately 50 mm Hg.12 Because the fetus is well adapted to low oxygen tensions, cyanosis is rarely present in newborn foals once adaption occurs, even those with low oxygen tensions. Although in many species the fetal blood oxygen affinity is greater than the maternal blood, in the equine fetus the O2 affinity of its hemoglobin is only approximately 2 mm Hg greater than the maternal blood as a result of lower levels of 2,3 diphosphoglycerate (DPG) compared with other species.13 The result is enhanced oxygen unloading in the equine fetus compared with others. DPG concentration increases after birth in the foal and reaches mature levels by 3 to 5 days of age. The major blood adaptation of the equine fetus to chronic hypoxia is an increase in PCV of up to 20%, increasing the oxygen content of the blood as compensation for decreased O2 delivery at the placenta.14 A higher than expected packed cell volume (PCV) in any newborn foal should alert the clinician to possible sequelae from chronic hypoxia. The presence of significant cyanosis that persists should prompt the clinician to thoroughly evaluate the foal for cardiac anomalies resulting in significant right to left shunting or separated circulations, such as transposition of the great vessels.
The body temperature of the foal at birth is similar to the dam’s temperature. Immediately after birth the healthy full-term neonate can effectively thermoregulate despite heat loss associated with wet hair coat and adverse environmental temperatures. Mechanisms of heat production in the healthy newborn foal include shivering, nonshivering thermogenesis, neuroendocrine stimulation, and behavioral processes.15 Newborn foals shiver within a few minutes of birth and continue to shiver for several hours when exposed to low environmental temperatures. Thyroid hormones and catecholamines stimulate the onset of nonshivering thermogenesis, which involves the oxidation of brown adipose tissue to release energy as heat.15 Behavioral thermoregulation involves the ability of the animal to modify its behavior to conserve or produce heat. Healthy foals maintain sternal recumbency to minimize heat loss but at the same time will increase heat production by physical activity, such as attempting to stand. A healthy foal should be nursing 1 to 2 hours after birth, thus relying on colostral fat, colostral glucose, and milk to provide sufficient energy to support metabolism such that fat stores are not seriously depleted. Premature foals are not able to maintain a steady rectal temperature because of inadequate heat production resulting from poor energy intake, diminished endogenous energy stores, lack of nonshivering thermogenesis associated with a lack of brown fat, and immaturity of the neuroendocrine system. Foals that suffer from a hypoxic insult, are septic, or are being treated with certain medications (e.g., phenobarbital) must be monitored and managed appropriately because they are unable to thermoregulate properly. In infants sedatives such as benzodiazepine affect the ability to thermoregulate; therefore sedation and general anesthesia used on the mare to correct a dystocia can have an impact on the newborn foal’s ability to thermoregulate.
The abdomen should be visually examined and palpated for abnormalities. Congenital scrotal hernias are noted shortly after birth, are usually unilateral, and are easily reduced when the foal is rolled onto its back. Intestinal strangulation is rare with scrotal hernias, and resolution of the hernia usually occurs spontaneously in 3 to 6 months. Inguinal hernias are evident 4 to 48 hours after birth and cause intermittent colic, depression, severe scrotal and preputial swelling, and edema, with skin excoriation and splitting caused by abrasion against the inside of the thigh. Inguinal hernias are classified as either indirect or direct, as described in the human literature. In indirect herniation the herniated intestine is situated inside the vaginal tunic and occupies the same space as the testis.16 Most indirect hernias are easily reduced and often resolve spontaneously as the foal ages and grows. In direct herniation the intestine herniates through a rent in the peritoneum and transverse fascia (and/or parietal vaginal tunic and scrotal fascia) and comes to lie subcutaneously (i.e., outside the common vaginal tunic, in the inguinal-scrotal region).16 Surgical intervention is often necessary for direct herniation because of the risk of incarceration of bowel. The exact cause is unknown, but it seems probable that inguinal hernias are a result of an increased intra-abdominal pressure during birth in which the intestinal loops may be forced through the vaginal ring into the vaginal cavity. Tearing of the parietal vaginal tunic could be caused by the limited space in the vaginal cavity. Umbilical hernias are a common defect in neonates and may result from congenital malformations of the umbilical region; infection; traumatic parturition; or opening of the umbilicus caused by straining, which results in increased intra-abdominal pressure. Occasionally, the umbilical defect may be so large that small intestine will eviscerate into the hernia sac. Small umbilical hernias resolve spontaneously, whereas larger hernias may require surgery to correct the defect. Auscultation of the abdomen provides an assessment of gastrointestinal motility and should be performed from the paralumbar fossa to the ventral abdomen, on both the right and left sides. Decreases in motility suggest ileus, which may be due to inflammatory, ischemic, or obstructive lesions. Increased motility occurs during the early stages of enteritis or intestinal obstruction.
The development of the gastrointestinal tract appears to be mostly complete in the neonate. The presence and distribution of the interstitial cells of Cajal (ICC), which generate pacemaker activity, are complete in the small intestine at birth; however, the development in the large intestine and distal colon continues after birth.17 This immaturity of the ICC at birth may contribute to retention of meconium in the distal colon and rectum in some neonatal foals. Other possible causes of meconium impaction include prematurity, sepsis, and asphyxia, or it can be secondary to prolonged recumbency, dehydration, or medications. Meconium, an accumulation of swallowed allantoic fluid, gastrointestinal secretions, and cellular debris, is generally black to brown and of firm to pasty consistency. Once meconium is passed, feces change to a lighter brown color and become softer. Evaluation of the quantity and quality of meconium is an important part of the physical examination, and the amount passed varies from foal to foal. Passage of meconium in utero is an indication of fetal distress during late gestation or parturition and should prompt a detailed assessment of the neonate. Aspiration of meconium results in severe pneumonia because of the caustic effect of the meconium on the respiratory epithelium.
Examination of the urogenital system includes evaluation of the external genitalia and the umbilicus. Many male foals have a persistent penile frenulum at birth, which resolves within a few days. When the cord is allowed to break naturally at birth, the umbilical vein and the urachus break at the navel stump and the umbilical arteries retract into their connective tissue sheath within the abdomen, closer to the bladder apex. Once the umbilical stump dries, it should remain dry. A moist stump or observation of urination from the umbilicus indicates a patent urachus, which is commonly found in foals that are recumbent for any reason. The neonate should be observed for the onset, frequency, and quantity of urine output. The mean time for first urination in the colt is 5.97 hours, and in the filly it is 10.77 hours; however, these times vary widely.18 Evidence of urogenital disease includes depression, straining to urinate, pigmenturia, and decreased urine output (oliguria). When clinical signs of urinary tract disease exist, the foal’s urine production should be monitored carefully and serum chemistries evaluated for azotemia and electrolyte disturbances, and a transabdominal ultrasound should be performed so that the clinician can examine the urogenital structures. The full-term neonatal foal has a glomerular filtration rate and effective renal plasma flow comparable with that of the adult.18 The status of the premature foal’s renal function has not been determined.
The musculoskeletal system should be closely evaluated for malformations or injuries secondary to the explosive nature of the equine birth. Congenital anomalies of the limbs include polydactylism, adactylia, defects in the hoof wall, and contractural deformities. Other congenital anomalies include brachygnathism, wry nose, cleft palate, torticollis, and scoliosis. Laxity in the neonate often manifests as weakness of the flexor tendons because of dysmaturity or prematurity, although full-term foals may exhibit a mild degree of fetlock laxity. In most foals the laxity will spontaneously resolve after a few days, but occasionally corrective trimming and shoeing are required. When the foal is malpositioned, injuries such as rib fractures and ruptures of the gastrocnemius muscle may occur. Rib fractures are common and occur even in apparently normal births. Rib fractures usually occur a few centimeters above the costochondral junction, with multiple fractures (4 to 12) commonly occurring in a straight line. Neonates with fractured ribs have soft tissue swelling over the fracture site, crepitus, and pain on palpation and occasionally respiratory distress resulting from pneumothorax or hemothorax. Rupture of the gastrocnemius muscle occurs during delivery if the hock is flexed and the stifle is forced into extension (i.e., the foal is “dog sitting”).19 This results in hyperflexion of the hock caused by tearing of the gastrocnemius muscle at the level of the femur. Most of the time these foals respond well to conservative management.
The righting reflex is present as the foal exits the birth canal, as is the withdrawl reflex. Cranial nerve responses are intact at birth, but the menace response may take as long as 2 weeks to fully develop. Lack of menace should not be considered diagnostic of visual deficits in the newborn foal. Within 1 hour of birth, the normal foal will demonstrate auditory orientation with unilateral pinna control. The normal pupillary angle is ventromedial in the newborn foal; this angle gradually becomes dorsomedial over the first month of life. Foals should begin attempting to stand shortly after birth and should be able to stand on their own within 2 hours of birth.20 The normal newborn foal will have a suck reflex shortly after birth and should be searching for an udder even before it stands. A normal foal is expected to suck from the dam unaided by 3 hours post partum; many foals will suck well before this time.
The gait of the newborn foal is hypermetric, and the stance is base wide.21 Extreme hypermetria of the forelimbs, usually bilateral but occasionally unilateral, has been observed in some foals and is associated with perinatal hypoxic-ischemic insults, but this gait abnormality usually resolves without specific therapy with a few days. Spinal reflexes tend to be exaggerated, while the crossed extensor reflex may not be fully present until 3 weeks of age.22 Foals exhibit an exaggerated response to external stimuli (e.g., noise, sudden visual changes, touch) for the first few weeks of life. Foals are not strongly bonded to their mothers for the first few weeks of life and will follow any large moving object, including other horses and people. Until they are several months of age, orphan foals will bond with surrogate mothers; their primary motivation appears to be appetite. Conversely, mares strongly bond with their foals shortly after parturition: The process begins once the chorioallantois is ruptured, and is driven more by olfaction and taste rather than by vision or hearing. Interference with this process, by medical intervention or excessive owner manipulation of the foal, can disrupt normal bonding and cause the dam to reject the foal.23
NEONATAL RESUSCITATION
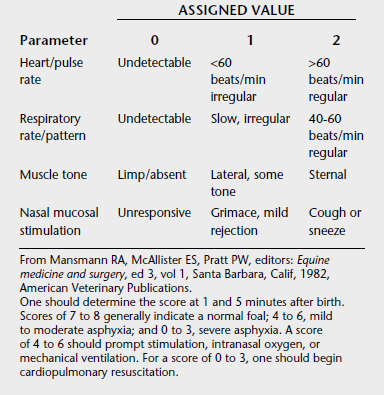
Most newborn foals make the transition to extrauterine life easily. However, for those in difficulty, immediate recognition of the condition and institution of appropriate resuscitation is of utmost importance. A modified Apgar scoring system has been developed as a guide for initiating resuscitation and assessing the probable level of fetal compromise (Table 21-2).1 One also must at least perform a cursory physical examination before initiating resuscitation in case there are serious problems (e.g., limb contracture, microophthalmia, hydrocephalus) that would make resuscitation a less humane choice.
The fetus is normally hypoxemic compared with the newborn foal, and this hypoxemia is largely responsible for the maintenance of fetal circulation by generation of pulmonary hypertension. The fetus responds to conditions producing more severe in utero hypoxia by strengthening the fetal circulatory pattern, and the neonate responds to hypoxia by reverting to the fetal circulatory pattern.2 During a normal parturition mild asphyxia occurs and results in fetal responses that pave the way for a successful transition to extrauterine life. If more than mild transient asphyxia occurs, the fetus is stimulated to breathe in utero; this is known as primary asphyxia.3 If the initial breathing effort resulting from primary asphyxia does not correct the asphyxia, a second gasping period occurs in several minutes, known as the secondary asphyxia response. If no improvement in asphyxia occurs during this period, the foal enters secondary apnea, a state that cannot be reversed except by resuscitation.
Therefore the first priority of neonatal resuscitation is establishing an airway and breathing pattern. The clinician should assume that foals not spontaneously breathing are in secondary apnea and should clear the airway of membranes as soon as the nose is presented. If meconium staining is present, the airway should be suctioned before delivery of the foal is complete and before the foal breathes spontaneously. One should continue to suction the trachea if aspiration of the nasopharynx is productive. Overzealous suctioning worsens bradycardia as it worsens hypoxia. Suctioning should be stopped once the foal begins breathing spontaneously because hypoxia will worsen with continued suction. If the foal does not breathe or move spontaneously within seconds of birth, one should begin tactile stimulation. If tactile stimulation fails to result in spontaneous breathing, the foal should immediately be intubated and manually ventilated using an Ambu bag or equivalent. Mouth-to-nose ventilation can be used if nasotracheal tubes and an Ambu bag are not available. The goal of this therapy is to reverse fetal circulation, and hyperventilation with 100% oxygen is the best choice for this purpose. However, recent evidence suggests that no clinical disadvantages are apparent when room air is used for ventilation of asphyxiated human neonates rather than 100% oxygen.4,5 Human infants resuscitated with room air recovered more quickly than those resuscitated with 100% oxygen in one study, as assessed by Apgar scores, time to the first cry, and the sustained pattern of breathing.6 In addition, neonates resuscitated with 100% oxygen exhibited biochemical findings reflecting prolonged oxidative stress, present even after 4 weeks of postnatal life, which did not appear in the group resuscitated with room air. Thus the current accepted recommendations for using 100% oxygen in the resuscitation of asphyxiated neonates require further discussion and investigation.7,8 Almost 90% of foals that need resuscitation respond to hyperventilation alone and require no additional therapy. One can initiate nasotracheal intubation while the foal is in the birth canal if the foal will not be delivered rapidly, such as with a difficult dystocia. This technique is “blind” and requires some practice but may be beneficial and lifesaving. Once spontaneous breathing is present, humidified oxygen should be administered by way of nasal insufflation at 8 to 10 L/min.
Cardiovascular support in the form of chest compression should be initiated if the foal remains bradycardic despite ventilation and a nonperfusing rhythm is present. The clinician should make sure that the foal is on a hard surface in right lateral recumbency with the topline against a wall or other support. Approximately 5% of foals are born with fractured ribs, and an assessment for the presence of rib fractures is in order before initiating chest compressions.9 Palpation of the ribs identifies many of these fractures, which usually are multiple and consecutive on one side of the thorax and located in a relatively straight line along the part of the rib with the greatest curvature dorsal to the costochondral junction. Auscultation over the ribs during breathing results in a recognizable click, identifying rib fractures that may have escaped detection by palpation. Ultrasonography is the diagnostic modality of choice when evaluating for the presence or absence of rib fractures in foals.10 Unfortunately, ribs 3 to 5 frequently are involved, and their location over the heart can make chest compression a potentially fatal exercise.11
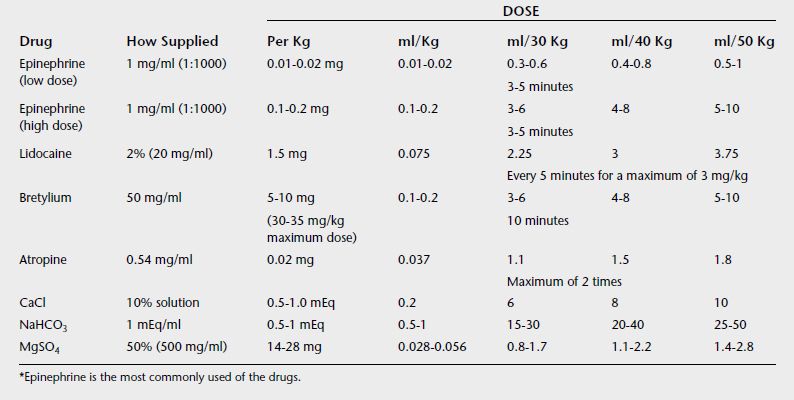
Drug therapy should be initiated if a nonperfusing rhythm persists for more than 30 to 60 seconds in the face of chest compression. Epinephrine is the first drug of choice (Table 21-3). Practitioners pose various arguments regarding the best dose and the best frequency of administration for resuscitation. However, most of the data are derived from human cardiac arrest studies and are not strictly applicable to the equine neonate because the genesis of the cardiovascular failure is different.12,13 Vasopressin is gaining attention as a cardiovascular resuscitation drug, and although the author has used this drug in resuscitation and as a pressor, experience is limited at this time and large doses of vasopressin may compromise gastrointestinal perfusion, according to some accounts.14–16 The author does not use atropine in bradycardic newborn foals because the bradycardia usually is caused by hypoxia, and if the hypoxia is not corrected, atropine can increase myocardial oxygen debt.17 The author also does not use doxapram in initial resuscitation because it does not reverse secondary apnea, the most common apnea in newborns. However, recent evidence suggests that doxapram may prove very useful for the treatment of persistent centrally mediated hypoventilation and deserves further study.18,19
Because birthing areas are generally cold, the foal should be dried and placed on dry bedding once resuscitation is complete. The fetus has some homeothermic mechanisms, but its size in relation to its mother and its position within her body means that it is in effect a poikilotherm. The body temperature of the foal generally reflects that of its environment, namely its dam, although the human fetal temperature directly measured at cesarean section, induction of labor, or during labor is approximately 0.5° C higher than the dam’s.20–22 Adaptation from poikilothermy to homeothermy normally takes place rapidly after birth. The fetus is capable of nonshivering thermogenesis, primarily through the oxidation of brown fat reserves, but this type of thermogenesis is inhibited in utero, probably by placental prostaglandin E2 and adenosine.22,23 Immediately after birth the foal must adapt to independent thermoregulation. Local physical factors, including ambient temperature and humidity, act to induce cold stress, and the newborn must produce heat by metabolic activity. In response to the catecholamine surge associated with birth, uncoupling of oxidative phosphorylation occurs within mitochondria, releasing energy as heat. This nonshivering thermogenesis is impaired in newborns undergoing hypoxia or asphyxiation and in those that are ill at birth. Infants born to mothers sedated with benzodiazepines are affected similarly, a consideration in the choice of sedative and preanesthetic medications in mares suffering dystocia or undergoing cesarean section.24–26 Heat losses by convection, radiation, and evaporation are high in most areas where foals are delivered, resuscitated, and managed, and one must take care to minimize cold stress in the newborn and the critically ill foal. Supplementary heat, in the form of radiant heat lamps or warm air circulating blankets, may be required.
Fluid therapy should be used conservatively during postpartum resuscitation, because the neonate is not volume depleted unless excessive bleeding has occurred. Some compromised newborn foals are actually hypervolemic. Fluid therapy for the neonate is discussed in more detail later in this chapter. Because the renal function of the equine neonate is substantially different from the adult’s, fluid therapy cannot simply be scaled down from adult therapy.27–29 If intravenous fluids are required for resuscitation and blood loss is identified, administration of 20 ml/kg of a non-glucose-containing polyionic isotonic fluid over 20 minutes (about 1 L for a 50-kg foal)—once intravenous access is established—can be effective. The author stresses non-glucose-containing polyionic intravenous fluids because hyperglycemia, but not hypoglycemia, immediately after fetal or neonatal asphyxia interfered with the recovery of brain cell membrane function and energy metabolism in neonatal piglets in one recent study.30 These findings suggest that post-hypoxic-ischemic hyperglycemia is not beneficial and might even be harmful in neonatal hypoxic-ischemic encephalopathy. Indications for this shock bolus therapy include poor mentation; poorly palpable peripheral pulses; and the development of cold distal extremities, compatible with septic or hemorrhagic shock (or both). The patient should be reassessed after the initial bolus, and additional boluses should be administered as necessary. Ideally, the clinician should follow up on blood pressures and ECG readings and initiate appropriate inopressor therapy if needed. Again, these procedures are discussed in detail later in the chapter.
Glucose-containing fluids can be administered after resuscitation at a rate of 4 to 8 mg/kg/min (about 250 ml/hr of 5% dextrose or 125 ml/hr of 10% dextrose) to the average 50-kg foal, particularly in the obviously compromised foal. This therapy is indicated to help resolve metabolic acidosis, to support cardiac output (because myocardial glycogen stores likely have been depleted), and to prevent postasphyxial hypoglycemia. Under normal conditions the fetal-to-maternal blood glucose concentration gradient is 50% to 60% in the horse, and glucose is the predominant source of energy during fetal development.31,32 Glucose transport across the placenta is facilitated by carrier receptors (glucose transporter [GLUT] receptors), and a direct relationship exists between maternal and fetal blood glucose concentration when maternal glucose is in the normal range.31 The GLUT receptors in the placenta are stereospecific, saturable, and energy independent.33 Although the enzyme kinetics for GLUT isoform 1 suggest that they are not saturable under conditions of euglycemia, equine maternal hyperglycemia results in increased fetal glucose concentration to a plateau point, likely caused by GLUT saturation.
At term the net umbilical uptake of glucose is 4 to 7 mg/kg/min, with most of the glucose being used by the brain and skeletal muscle.34–36 The fetus develops gluconeogenesis only under conditions of severe maternal starvation. A certain percentage of the delivered glucose is used to develop large glycogen stores in the fetal liver and cardiac muscle in preparation for birth, and at birth the foal liver produces glucose at a rate of 4 to 8 mg/kg/min by using these stores. Fetal glycogen stores also are built using the substrates lactate, pyruvate, and alanine; fetal uptake of lactate across the placenta is about half that of glucose.31,37 The transition to gluconeogenesis, stimulated by increased circulating catecholamine concentration from birth and by stimulation of glucagon release at the time the umbilical cord breaks, takes 2 to 4 hours in the normal foal, and glycogenolysis supplies needed glucose until feeding and glucose production are accomplished.38 In the challenged foal glycogen stores may have been depleted and gluconeogenesis delayed, so provision of glucose at rates similar to what the liver would normally produce during this period is requisite.
PERSISTENT PULMONARY HYPERTENSION
Pulmonary vascular resistance falls at delivery to about 10% of fetal values, and pulmonary blood flow increases accordingly.1 Early in the postnatal period these two changes balance each other, and mean pulmonary and systolic pressures remain increased for several hours. Systolic pulmonary pressures can remain equivalent to systemic pressure for up to 6 hours of age in human infants, although diastolic pulmonary pressures are well below systemic diastolic pressures by 1 hour.2 Mean pulmonary artery pressures fall gradually over the first 48 hours.3 The direct effects of lung expansion and increasing alveolar oxygen tension probably provide the initial stimulus for pulmonary arteriolar dilation and partly result from direct physical effects, but vasoactive substances are released in response to physical forces associated with ventilation (e.g., prostacyclin).1 Other vasoactive mediators thought to play a role in regulating pulmonary arteriolar tone include NO, prostaglandins D2 and E2, bradykinin, histamine, endothelin-1, angiotensin II, and atrial natriuretic peptide. The increase in alveolar and arterial oxygen tensions at birth is required for completion of resolution of pulmonary hypertension. Much of this increase is thought to be mediated by NO, evidence for this being the parallel increase during gestation of the pulmonary vasodilation response to hyperoxia and the increase in NO synthesis.4 However, inhibition of NO synthesis does not eliminate the initial decrease in pulmonary artery resistance occurring because of opening of the airways.5
When these mechanisms fail, PPH can be recognized. Right-to-left shunting within the lungs and through patent fetal conduits occurs and can result from many factors, including asphyxia and meconium aspiration, but in many cases the precipitating trigger is unknown. Inappropriately decreased levels of vasodilators (NO) and inappropriately increased levels of vasoconstrictors (endothelin-1) currently are being examined as potential mechanisms. One study of oxygen-dependent clone calves revealed increased neonatal and maternal endothelin concentrations.6 Chronic in utero hypoxia and acidosis may result in hypertrophy of the pulmonary arteriolar smooth muscle.7 In these cases reversal of PPH can be difficult and cannot be achieved rapidly.
Treatment of PPH is twofold: abolishment of hypoxia and correction of the acidosis, because both abnormalities only bolster the fetal circulatory pattern. Initial therapy is provision of oxygen intranasally at 8 to 10 L/min. Some foals respond to this therapy and establish neonatal circulatory patterns within a few hours. Failure to improve or worsening of hypoxemic respiratory failure after intranasal oxygen administration should prompt intubation and mechanical ventilation with 100% oxygen. This serves two purposes, one diagnostic and one therapeutic. Ventilation with 100% oxygen may resolve PPH and, if intrapulmonary shunt and altered ventilation-perfusion relationships are causing the hypoxic respiratory failure, arterial oxygen tension (Pao2) should exceed 100 mm Hg under these conditions. Failure to improve Pao2 suggests PPH or large right-to-left extrapulmonary shunt caused by congenital cardiac anomaly. The vasodilators prostacyclin and telazoline cause pulmonary vasodilation in human infants with PPH, but the effects on oxygenation vary and the adverse effects (e.g., tachycardia, severe systemic hypotension) are unacceptable.8 Recognition of NO as a potent dilator of pulmonary vessels has created a significant step forward in the treatment of these patients, because inhaled NO dilates vessels in ventilated portions of the lung while having minimal effects on the systemic circulation.9 According to presently available evidence, use of inhaled NO in an initial concentration of about 20 ppm in the ventilatory gas seems reasonable for term and near-term foals with hypoxic respiratory failure and PPH that fails to respond to mechanical ventilation using 100% oxygen alone.9,10
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree