CHAPTER 101 Diagnosis of Pregnancy
Early and accurate identification of pregnant and nonpregnant sows and gilts has a strong potential to increase reproductive efficiency of commercial swine farms. Detection of returns to estrus after mating, ultrasound techniques, and several other methods have been used for pregnancy diagnosis1; however, for various reasons, only three or four techniques currently are used in commercial farms.
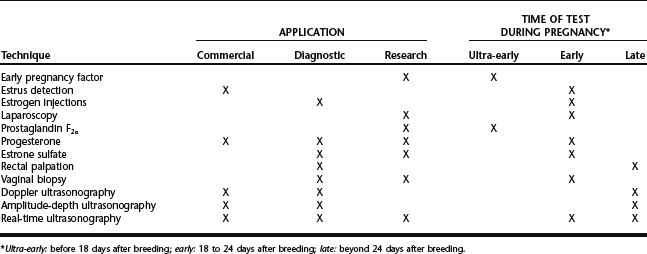
An ideal pregnancy detection technique is not available, and most producers use either a combination of techniques or one technique that apparently fulfills their requirements. Some methods are restricted to research applications, and it is unlikely that these methods will be adapted for on-farm use. This chapter reviews the techniques for pregnancy diagnosis in swine; the practical applications, or lack thereof, are emphasized for each technique (Table 101-1). For most procedures, sensitivity (ability to detect pregnant animals, representing the proportion of pregnant animals that test positively) and specificity (ability to detect nonpregnant animals, representing the proportion of nonpregnant animals that test negatively) are used to assess accuracy.
DETECTION OF ESTRUS
A common pregnancy detection technique is observation of the sow for failure to return to estrus after mating. This technique is based on the premise that nonpregnant sows will return to estrus within 17 to 24 days after breeding. Detection of estrus is improved if the sow’s behavior is observed in the presence of a boar.2 Thus, this technique requires gestation facilities that are designed to allow daily fenceline contact between boars and sows, or the placement of the boar and sow in the same pen each day. Injections of estradiol or estradiol plus testosterone will enhance estrous behavior in sows that are not pregnant3; however, these steroids are not approved for use in swine in the United States.
An early study reported an accuracy of 39% for the detection of return to estrus between 19 and 25 days after mating.4 By contrast, daily estrus detection throughout gestation enabled a 98% accuracy in predicting farrowing rate.5 Albeit rarely seen, a false negative result can be obtained if a pregnant sow shows spontaneous estrus.5 False positive results occur in the following scenarios:
HORMONE CONCENTRATIONS
Prostaglandin F2α
The endometrium of the nongravid uterus secretes PGF2α into the uterine vein between days 12 and 15 of the cycle, thereby inducing regression of the corpora lutea.6 When viable embryos are present, PGF2α is not secreted into the uterine vein.7 The prostaglandin pregnancy test is based on the principle that if serum concentrations of PGF2α are low (less than 200 pg/ml) or undetectable between days 13 and 15 after mating, the sow can be assumed to be pregnant.8 Routine blood sample collection, without the use of indomethacin or other cyclo-oxygenase inhibitors, causes release of PGF2α from blood constituents. This PGF2α release leads to obvious problems with interpretation of serum PGF2α concentrations; however, prostaglandin metabolites are more stable, and serum metabolite concentrations are reasonable indicators of prostaglandin concentrations.
The prostaglandin pregnancy test has approximately 90% sensitivity and 70% specificity. Accuracy is lower when animals have delayed returns to estrus, or when fetal death occurs and sows manifest pseudopregnancy. This diagnostic method can be used during early pregnancy, but its unreliability in the detection of nonpregnant animals and the necessity of extensive laboratory procedures limit its practical application.
Progesterone
Maintenance of the corpus luteum (CL) is the result of a blastocyst “signal” that is produced at 10 to 12 days after mating.9 The blastocyst-induced maintenance of corpora lutea causes serum progesterone concentrations to remain high (greater than 5 ng/ml) throughout pregnancy. Thus, serum concentrations of progesterone are high in pregnant sows and gilts during the expected time of return to estrus and low (less than 5 ng/ml) in bred sows and gilts that fail to conceive.10
The interestrus interval for sows of various parities ranges from 17 to 24 days, with a mean of 20 to 21 days and a mode of 20 days.11 Therefore, the optimal time to obtain blood samples for progesterone determinations is from 17 to 20 days if nonconceiving sows and gilts are to be identified before the time they return to estrus. The serum concentration of progesterone that most accurately discriminates the nonpregnant from the pregnant sow or gilt has not been determined. Concentrations of 4, 5, 7, 7.5, and 9 ng of progesterone/ml of serum were used to discern pregnancy status5,10; a concentration of 5 ng/ml is most commonly used.
The progesterone pregnancy test has greater than 97% sensitivity at 17 to 24 days, but specificity ranges from 60% to 90%.5,12 False positive results are common when nonconceiving sows and gilts have delayed or irregular returns to estrus, and when nonpregnant sows and gilts are anestrous as a result of cystic ovarian disease.5 False negative results may be the result of laboratory error because it is assumed that greater than 4 ng of progesterone/ml of serum is required for pregnancy maintenance in swine.13
Commercial enzyme-linked immunosorbent assays (ELISAs) to measure blood concentrations of progesterone in swine can be used on farms or in veterinary clinics, thereby reducing the need for laboratory-based radioimmunoassays. The necessity of collecting blood is a significant limitation of this method; however, methods to quantitate fecal progestins were evaluated for monitoring reproductive function in swine,14 dogs,15 and ruminants.16 It was evident that the extraction and assay procedures are feasible alternatives to blood progesterone assays. Despite the potential use of fecal progestin determinations, direct applications of this methodology for pregnancy diagnosis have not gained popularity, nor have they been evaluated on a large scale in commercial pig production.
Estrone Sulfate
A high proportion of fetal estrogens is secreted from the uterus into the maternal circulation as estrone sulfate.17 Estrone sulfate initially appears in the maternal circulation at approximately 16 to 20 days and rises linearly to peak levels between 25 and 30 days before decreasing to a nadir at 35 to 45 days.18 A second increase in estrone sulfate occurs concomitantly with increases in other estrogens, commencing at 70 to 80 days and continuing until farrowing.
Urinary and serum estrone sulfate concentrations have been investigated for applicability as pregnancy tests.5,19,20 A 10-fold to 100-fold increase in maternal estrone sulfate concentrations typically occurs between 25 and 30 days after coitus in sows that conceive.21 Because estrone sulfate increases during early and late pregnancy, determination of estrone sulfate levels has potential applications as an early pregnancy diagnosis test and as a confirmatory test later during pregnancy. Parity, season, and day of pregnancy when blood was collected have some effect on estrone sulfate concentrations.
Serum estrone sulfate concentrations greater than 0.5 ng/ml are indicative of pregnancy, whereas concentrations less than 0.5 ng/ml are suggestive of nonpregnant status.5,19 The estrone sulfate pregnancy test has greater than 97% sensitivity and greater than 88% specificity when serum samples are collected between 25 and 30 days of pregnancy.5 False positive results may be attributable to a transient increase of estrone sulfate concentrations in some sows and gilts during proestrus.18 False negative results were obtained in sows or gilts with a premature or delayed rise in estrone sulfate concentrations22 or in sows and gilts that had less than 4 pigs in a litter.5 As with other early tests of pregnancy, animals may be correctly diagnosed as pregnant but subsequently will fail to farrow if the fetuses die after the test has been conducted.
Pseudopregnant sows frequently retain the endocrine function of the corpora lutea for prolonged periods and may appear to be pregnant even though they no longer have viable fetuses. Other sows experience loss of their pregnancies, followed by development of acyclic, anovulatory ovaries or cystic ovaries, and similarly fail to show estrus for various time periods. Serum concentrations of estrone sulfate in pseudopregnant animals were similar to those in females that had not been mated and distinctly less than those in pregnant sows.23
Urinary concentrations of estrone conjugates also have been used to predict pregnancy and to diagnose fertility problems.20 for these investigations, urine samples were obtained through the use of vaginal sponges. It should be noted that the glucuronide conjugate of estrone, rather than the sulfate form, was measured; thus, different quantitative procedures were required.
Early Pregnancy Factor
Early pregnancy factor (EPF) activity is dependent on the presence of two components: EPF-A and EPF-B. Factor A is formed in the uterus during estrus and pregnancy, whereas EPF-B is produced in the ovary and is associated only with pregnancy. The production of EPF-B is a result of a combined action of endocrine signals from the pituitary and the zygote.24 Serum concentrations of EPF peak 24 to 48 hours after fertilization and exhibit a polyphasic pattern throughout gestation.25
Detection of EPF, based on a rosette inhibition test, is time-consuming and cumbersome and possesses other limitations. Various applications of EPF detection have been proposed for embryo transfer programs and assessment of infertility and early embryonic survival.26 Several investigations indicated that EPF detection was a useful technique to evaluate human infertility and embryonic loss; however, little evidence is available to substantiate the use of EPF detection in commercial swine production. One study indicated that the rosette inhibition test was not quantitative or suitable for pregnancy diagnosis in swine.27
PHYSICAL METHODS
Radiography
Radiography is a seldom-used method for pregnancy diagnosis in swine. In research studies, it was used after the sixth week of pregnancy,28 when the fetal skeleton begins to calcify. Fetal age, viability, and abnormalities were determined with radiography.29 Equipment costs, potential health hazards to users, and the impracticality of radiography in production facilities render this technique unsuitable for pregnancy diagnosis in commercial swine.
Rectal Palpation
Pregnancy diagnosis by rectal palpation of the sow has been demonstrated to be practical and highly accurate.30 Sows in the relevant study were examined while standing in gestation crates or pens, or while tethered. The technique involves examination of the cervix and uterus, together with palpation of the middle uterine artery to assess its size, degree of tone, and type of pulse. Before 21 days of gestation, rectal palpation had a 30% sensitivity, but the sensitivity increased to 75%, 94%, and 100% in animals at 21 to 27, 28 to 30, and 60 days to term, respectively.30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree