Chapter 44 Diagnosis of Aspergillosis in Avian Species
Invasive aspergillosis presents a diagnostic dilemma in mammalian and avian species.3,19,20,25,26 Traditional techniques such as routine hematology and plasma biochemistry may complement radiographic investigations but results rarely provide a definitive diagnosis. Endoscopy is often available but is invasive and may not be readily used on severely ill birds.17 Serologic test panels have been recently implemented and described for use in avian species.* This chapter summarizes this information as well as these and other studies undertaking a multidimensional diagnostic approach to detecting this disease.
Antibody Detection
In clinical veterinary medicine, the value of serodiagnostic testing to detect antibody levels has been described in horses, dogs, and birds.† Primary methodologies available at some veterinary laboratories include agarose immunodiffusion (AGID) and enzyme-linked immunosorbent assay (ELISA). For AGID, a positive reaction is displayed in the form of a precipitin line of antibody-antigen complexes in the agarose media. This test is qualitative or semiquantitative at best. The method offers no restrictions regarding the species from which the specimen is derived because no secondary reagents are needed. However, because the interpretation is visual, the sensitivity of the test is limited. In contrast, ELISA offers increased sensitivity and specificity although its use is restricted to specimens for which secondary reagents are available (e.g., anticanine immunoglobulin G [IgG]). In the United States, AGID remains the more commercially available method. In 1994, Brown and Redig4 described the implementation and use of ELISA in companion birds and raptors. This assay was commercially available for many years, as is the ELISA methodology currently offered by the University of Miami, which was described in a recent publication.9
Martinez-Quesada and coworkers have demonstrated antibody titers in pigeons that were immunized with Aspergillus fumigatus extracts.24 In this study, IgG titers were demonstrable from 14 days postimmunization, with a peak at 63 days. Titers were demonstrable, albeit lower, through 210 days, but booster immunizations resulted in the production of high levels of antibody. A high seroprevalence of antibody has been described in captive and wild penguins by ELISA.13,15,27 German and associates13 have observed that 93% (of 61 penguins) were seropositive and serostatus could not be correlated with clinical disease. This was similar to another study of captive penguins, in which the high seroprevalence prompted the authors to suggest that most penguins are infected or colonized by Aspergillus spp.27 In an experimental model of infection in two duck species, Graczyk and colleagues concluded that the applicability of the antibody ELISA is low.14 These studies suggest that antibody levels may be high in many avian species with a normal clinical condition. These levels may be long-lived and perhaps subject to restimulation through continued environmental exposure to Aspergillus spp.
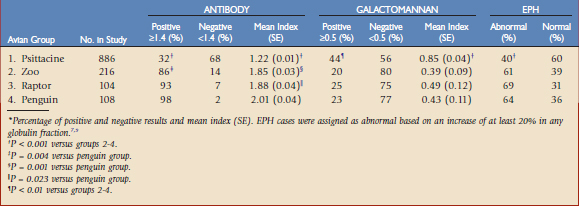
In a recent study that included a large serosurvey of different avian species (N = 1314), Cray et al.9 reported that most avian species grouped as zoo species (mostly land and waterfowl species), raptors, and penguins were seropositive for antibody to Aspergillus spp. (Table 44-1). This was in contrast to the psittaciform group, which only had a 32% positive serostatus. Additional data was gathered on 303 of the submissions, which enabled them to be categorized as presumptive nonaspergillosis, probable (on the basis of clinical signs and response to treatment), or confirmed (on the basis of histology, culture, and/or polymerase chain reaction [PCR] assay on necropsy or biopsy tissues). Most of these cases represented psittaciform species. This data is presented in Table 44-2 and shows no significant differences between the clinical groups (P = 0.059). The results are consistent with a small study of seven psittaciform birds with confirmed aspergillosis.17 Interestingly, when the data was examined as percentage positive cases (index ≥1.4), 69% of the presumptive normal birds were negative versus only 42% of the confirmed cases (P = 0.022).
Although the antibody ELISA may have limited value as a single serodiagnostic test for avian species, it may still have some applicability. Whereas antibody serostatus may be of small consequence in zoo species, raptors, and penguins, with most birds being seropositive, most psittaciform cases in this study were seronegative.9 Therefore, the presence of antibody in this species would be considered unusual and perhaps clinically remarkable. In addition, positive antibody serostatus was more often observed in those birds with confirmed infection, although the actual antibody index was not a strong indicator of infection. That is, the qualitative rather than the quantitative result may be helpful in some avian species.
It has been proposed that cell-mediated immunity, especially Th1 responses, are important in modulating macrophage responses in aspergillosis.3 Given this premise, it would be expected that antibody responses, which are mediated via the Th2 pathway, would not be a primary effector mechanism in the proper response to Aspergillus spp., although it may provide help in the initial resistance to infection. Antibody titers may also vary with the health status of the patient. Low levels of antibody reactivity may be present because of the immunosuppressive properties of toxins secreted by Aspergillus spp.
Antigen Detection: Galactomannan
Galactomannan is a major component of the fungal cell wall that is released during the growth of hyphae. With invasive infection, this antigen may be found in the blood circulation. A commercially available ELISA that is reactive to β-(1-5)-galactofuranose has been widely implemented in human clinical pathology laboratories for the detection of invasive aspergillosis in immunosuppressed patients in whom fungal disease is opportunistic and often associated with high rates of morbidity and mortality.26 This population, given the severe immunosuppression and presence of chronic disease, rarely produce antibody to Aspergillus spp., making an antigen detection test a potentially ideal method. The ELISA is based on the sandwich method and, because it is based on internal positive and negative controls, the result is reported as an index with no units. Because of changing guidelines for human use, many early studies (through 2006) often used an index of 1.5 or 1.0 as a positive cutoff value. To improve the sensitivity of the assay, the guidelines were later changed to 0.5. When reviewing the literature, especially with reference to sensitivity and specificity, it is important to reference the cutoff level that was used in the respective studies.
In addition to the validation of the galactomannan ELISA in human and animal models of invasive aspergillosis (including rodents, guinea pigs, and rabbits), the assay has been studied in horses, cows, dogs, and avian species.* In dogs with aspergillosis, Garcia and coworkers12 reported a high frequency of antibody as measured by ELISA versus a variable presence of galactomannan. The latter was consistently observed only in a dog with systemic infection and a dog with a severe bronchopneumonia that progressed to death. In horses that often demonstrated antibody reactivity to Aspergillus spp., galactomannan was detected in a case with systemic aspergillosis but not in those with guttural pouch infection, an important point to note.16 In cows experimentally infected with A. fumigatus as well as in cows with naturally occurring systemic infection, galactomannan was detected.18 Notably, this study also reported that the level of galactomannan likely is associated with the burden of infection, a finding that had also been suggested by reports of human cases and in laboratory animal models.26
In a study of experimental infection in ducks using an early bench top version of the galactomannan assay, Graczyk and colleagues14 were the first to report the possible high predictive value for antigen detection in invasive aspergillosis. Le Loc’h and associates21,22 reported a specificity of 86% and positive predictive value of 75% for the commercial galactomannan ELISA in a collection of specimens from psittaciform species with suspected infection. However, using a positive index cutoff of 1.0, they observed a low sensitivity of 30%. In those birds with the poorest general health, the galactomannan values were observed to be the highest (index >3.5). In addition, birds showing signs of invasive infection were twice as likely to be positive than those with only respiratory signs. The galactomannan ELISA was also evaluated in a large study of falcons.1 Comparing confirmed cases and confirmed aspergillosis-negative cases, a sensitivity of 12% and specificity of 85% were reported. This study was also conducted with the higher 1.0 index cutoff level. Given this data analysis, it was concluded that the assay should not be used as a screening test for aspergillosis in falcon species.
Galactomannan was also examined in a large study by Cray and coworkers.7 In a general serosurvey using a positive cutoff index of 0.5, 20% to 25% of zoo species, raptors, and penguins were found to be positive for circulating galactomannan (see Table 44-1). This was significantly lower than that observed in specimens from the psittaciform group. In the extended study of presumptive nonaspergillosis, probable, and confirmed cases (as described earlier), significantly higher levels of galactomannan were observed in the probable and confirmed groups (see Table 44-2). The sensitivity was 67% and the specificity was 73%. When the data were reanalyzed with a positive cutoff level of 1.0, the sensitivity decreased to 39% and the specificity increased to 83%. These results are consistent with those reported by Arca-Ruibal and colleagues1 and Le Loc’h and associates.21,22 Given the observation of the mean value of the presumptive nonaspergillosis group to be 0.64, the University of Miami currently uses the value of 0.7 or higher as the positive cutoff index for avian samples.
The galactomannan ELISA is used in human patients with reservation. Some cross-reactivity has been reported with other microbial antigens, including Penicillium spp. and Histoplasma capsulatum.26 In addition, positive indices may be observed in clinically normal individuals exposed to galactomannan in the environment via food, drink, intravenous hydration or nutrition fluids, use of piperacillin or other beta-lactam antibiotics, or environmental aerosols. Antigenemia is also considered to be variable under any infectious process. This has been observed in humans and in dogs in which periodic testing has been performed.12 Biologic factors, including the site of infection and the microenvironment at the site, may help or hinder the presence of galactomannan in the blood circulation. In a report of several confirmed cases of aspergillosis, Cray and coworkers6 found negative galactomannan levels in a cockatoo with infection limited to a tracheal granuloma. This is in contrast to two cases with lung and air sac involvement, in which indices ranged from 5.3 to 6.7. In addition, recent exposure to antifungal agents as well as the presence of anti-Aspergillus antibodies may affect the galactomannan levels. The latter may be especially problematic in use of the assay in avian species. As reported, zoo species, raptors, and penguins often have high circulating levels of antibody. The presence of antibody may effectively clear the circulating galactomannan such that it is not detected by the ELISA. This may account for the data in the current study. In psittaciform species, in which antibody reactivity is less frequently observed, higher levels of galactomannan were observed in the serosurvey versus zoo species, raptors, and penguins (see Table 44-1). I have frequently observed this dichotomy of antibody and galactomannan results in the laboratory. That is, very high-level antibody indices are often accompanied by negative galactomannan indices. Thus, although the predictive value of an individual antibody ELISA result is of questionable value, it is important to know the level of antibody when interpreting the galactomannan result.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree