Intervention [reference]
Agea (weeks)
Diet
Brachiocephalic artery (%)
Carotid artery (%)
20
WTD
8
0
Age [5]
42–50
Chow
66–75
n.d.
Collar [7]
52
WTD
n.a.
8
Ligation + collar [6]
13
WTD
n.a.
100
Irradiation 14y [3]
44–52
Chow
n.d.
85
Collar + Ad.MMP9 [7]
18–20
WTD
n.a.
90
adMMP bone marrow transplant [8]
52
chow
50
n.d.
Thrombomodulinpro/pro + collar [10]
18
WTD
n.d.
unk
Fibrillin1C1039G+/− [4]
20
WTD
88
92
The detection of intraplaque hemorrhage, be it in mice or man, involves histology to identify the components of intraplaque hemorrhage. This chapter describes the protocols for hematoxylin and eosin (HE) and Martius Scarlet Blue (MSB) staining, able to discriminate erythrocytes and fibrin, and the Perl’s Prussian blue staining identifying ferric iron. The protocols in this chapter should preferably be used together to achieve maximal reliability in the detection of intraplaque hemorrhage.
1.1 Hematoxylin and Eosin
The widely used combination of hematoxylin and eosin allows the discrimination between nuclei and cytoplasm/connective tissue, respectively. Hematoxylin itself does not stain nuclei, but rather its oxidation product hematein. In this protocol, Meyer’s hematoxylin is used, which is chemically oxidized by sodium iodate. Hematein is anionic, as is nuclear chromatin, and will thus only stain nuclei in the presence of a mordant, in this case aluminium salt. The mordant/metal cation will positively charge hematein, providing a net charge able to bind and stain the anionic nucleic acids in chromatin in an acidic environment. The color change from red to blue occurs in alkaline pH, which is achieved by washing in a weak alkaline solution, such as tap water.
Eosin distinguishes the cytoplasm of different cell types and types of connective tissues by staining them different shades of red (Table 2). Fibrin is very acidophilic and is thus strongly stained by eosin and appears as distinctly pink (Fig. 1a, b) [11].
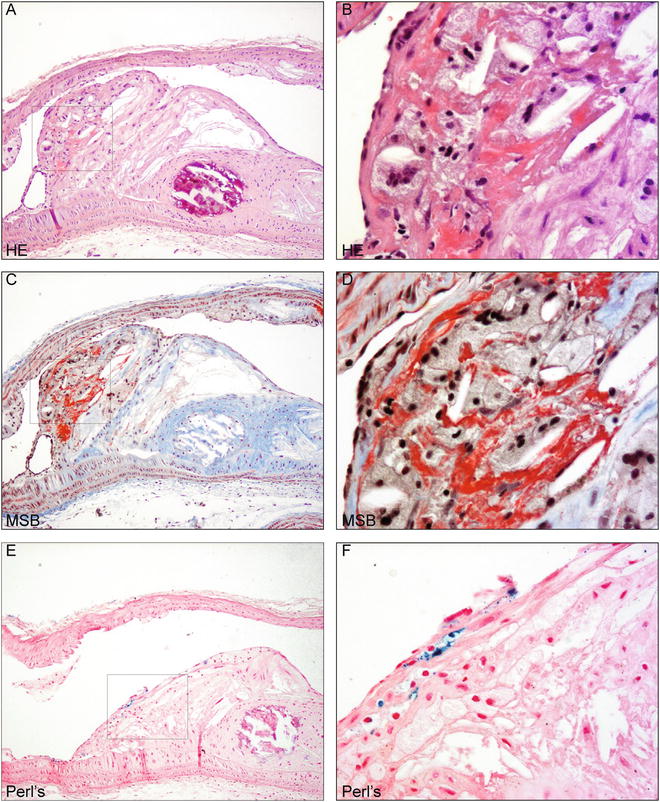
Table 2
Colors identifying tissue components after each staining
Staining | |||
|---|---|---|---|
Structure | HE | MSB | Perl’s |
Nuclei | Blue | Black | Red |
Cytoplasm | Varying shades of pink | Yellow | Light red |
Erythrocytes | Orange/red | Yellow | Red |
Fibrin | Fuchsia | Red | n.a. |
Collagen | Pink | Blue | n.a. |
Iron | Brown-yellow | n.a. | Blue |

Fig. 1
Murine intraplaque hemorrhage detection using HE, MSB, and Perl’s ferric iron (a). Atherosclerotic plaque in the aortic arch of ApoE−/− mice fed chow for 30 weeks after single irradiation dose of 14y [3] stained with hematoxylin and eosin. Bright pink indicates fibrin. Magnification 100×. Boxed region is shown in higher magnification in (b). Detail of fibrin indicating intraplaque hemorrhage. Magnification 400×. (c). Section of same plaque stained with MSB protocol detecting fibrin in bright red. Magnification 100×. Boxed region is shown in higher magnification in (d). Detail of fibrin indicating intraplaque hemorrhage. Magnification 400×. (e). Section of same plaque stained with Perl’s Prussian blue for ferric iron detection in blue, counterstained with nuclear fast red. Magnification 100×. Boxed region is shown in higher magnification in (d). Detail of iron in macrophage indicating intraplaque hemorrhage. Magnification 400×
1.2 Martius Scarlet Blue
The MSB procedure includes application of Martius yellow, Brilliant Scarlet red, and Alcian blue dyes to easily distinguish erythrocyte cytoplasm, fibrin, and collagen, respectively, while nuclei appear black (Table 2, Fig. 1) [11]. Nuclei are first stained black with Weigert’s iron hematoxylin, a solution allowing stable and acid-resistant nuclear detection if compared to alum hematoxylins, such as Meyer’s hematoxylin. Martius yellow is then applied together with phosphotungstic acid in alcoholic solution to stain the cytoplasm of erythrocytes yellow. Mature fibrin will then be stained by brilliant crystal scarlet (Fig. 1c, d). Phosphotungstic acid will subsequently fix the dye in fibrin fibrils while removing any red stain from the collagen and blocking staining of muscle, collagen and connective tissue. Aniline blue subsequently stains collagen [11]. The MSB staining is selective for fibrin, but not entirely specific. Bone tissue, granulae in Paneth’s cells, and other cytoplasmic vesicles may stain. The combination of the three histological stains described in this chapter will resolve the detection of fibrin.
1.3 Perl’s Prussian Blue for Ferric Iron
Perl’s iron visualizes ferric iron ions in hemosiderin, which is hydrated, protein-bound iron oxide (Fe(OH)3). Hemosiderin is always located within cells and an intraplaque hemorrhage can result in hemosiderin deposits in the macrophages. In unstained or HE-stained tissue sections, hemosiderin presents as yellow-brown, coarse-grained pigment, which is more easily identified upon Perl’s Prussian blue staining [11]. Ferric iron in hemosiderin reacts with potassium ferrocyanide to yield insoluble blue ferric ferrocyanide precipitate (Prussian Blue, Fig. 1e, f). This reaction takes place in an acidic milieu to liberate iron from its protein carrier, such as hemoglobin, as follows: 4Fe(OH)3 + 3K4Fe(CN)6 + 12HCl Fe4[Fe(CN)6]3 + 12KCl + 12H2O [11].
2 Materials
1.
Formalin-fixed, paraffin-embedded murine arterial sections (4–5 μm) on adhesive, silane-coated glass slides (see Note 1 ).
2.
Xylene.
3.
Alcohol 100 %.
4.
Alcohol 96 %.
5.
Alcohol 70 %: 700 ml alcohol 100 %, 300 ml distilled water.
6.
Alcohol 50 %: 500 ml alcohol 100 %, 500 ml distilled water.
7.
Distilled water.
8.
Tap water.
10.
Rack to place glass slides in and transfer between containers.
13.
Microscope.
14.
Timer.
16.
Stir plate.
17.
Stir Bar.
18.
Flasks.
19.
Filter paper or equivalent for blotting or filtration.
20.
Funnel.



