CHAPTER 31 Dermatophilosis
Dermatophilosis is a common pustular and crusting skin disease of horses caused by Dermatophilus congolensis.1 Various names have been used to describe this disease in horses, including streptothricosis, cutaneous actinomycosis, rain rot, mud fever, and dew poisoning.
ETIOLOGY AND EPIDEMIOLOGY
Dermatophilus is a gram-positive, non–acid-fast, facultative anaerobic, branching actinomyces.2 Genotypic and phenotypic variation between isolates has been demonstrated.3–5 Dermatophilus has a distinct life cycle and exists in two morphologic forms, hyphae and zoospores.6,7 Hyphae are composed of filaments that break into coccoid cells. These cells mature into flagellated zoospores, which represent the infective stage.8
The natural habitat of Dermatophilus is unknown.1,9 Many attempts to isolate the organism from the soil have failed,10 even when soil samples were collected from the immediate environment of diseased animals.11 In one study, however, Dermatophilus was isolated from the soil, and its survival appeared to depend on the type of soil and the water content.12 Organic matter has a protective effect on the microorganism. Because the pathogenicity of Dermatophilus congolensis was preserved in soil, it is hypothesized that soil could act as a temporary reservoir for the organism. Dermatophilus can also survive in the skin of animals that are clinically normal, potentially acting as source of infection once favorable conditions are present.13,14 Crusts from affected animals represent an important source of contagion for spreading lesions on the same animal and possibly the infection of other animals in the same herd.15
PATHOGENESIS
Establishment of infection with D. congolensis appears to depend on a variety of factors, including the virulence of the strain, the general health of the animal, skin trauma, and moisture.16 Zoospores germinate, producing hyphae under favorable conditions. Hyphae penetrate the epidermis and spread from the initial area, triggering an inflammatory response.17,18 Coccal cells are released from crusts to establish new sites of infection.
Virulence of the Strain
Strain differences in virulence affect the ability of D. congolensis to establish infection in the host.19–22 Isolates from the same animal species or from the same geographic location are not always closely related genetically.23 More specifically, variability may be observed in hemolytic activity on blood agar,24 phospholipases,25 proteases and lipases,4,26 mucoid nature of colonies, motility, flagella density and polarity, capsule width, restriction enzyme profiles of bacterial deoxyribonucleic acid (DNA), protein electropherotype, and carbohydrate content. Hemolytic activity and enzyme activity of proteins and lipids appear to be important determinants of infectivity.5 Dermatophilus also produces ceramidases,27 which are thought to play a role in the pathogenesis of the disease through their protective and cell regulatory functions in the epidermis.
The extracellular products of Dermatophilus have proteolytic activities,28 including the ability to digest keratin, which could play an important role in the establishment of the infection.29,30 Zoospores are the most likely source of extracellular proteases, and their ability to function at a wide pH range enables the bacterium to adjust to the pH variations of inflamed skin.30 Because the stratum corneum is filled with lipids and proteins,31 it is reasonable to speculate that D. congolensis may use lipases and proteases to penetrate this barrier. Proteases may have a role in acquisition of nutrients and may initiate or inactivate host inflammatory protease cascades or cytokines.
Skin Trauma and Insects
Dermatophilus is unable to infect intact skin but can readily infect traumatized skin.32 Various insects and ticks play a role as vectors for the transmission of Dermatophilus and as causes of trauma, which makes the skin more susceptible to the infection.33,34 The inflammatory response triggered by biting flies provides a suitable growth medium for Dermatophilus.
Climatic Conditions and Host Factors
Moisture causes the release of infective zoospores of D. congolensis,1 with resultant increased incidence of this disease during wet seasons and heavy rainfall.9,32,35,36 Rainfall contributes to the infection in several ways, such as increasing the population of hematophagous flies, which cause skin damage and initiate inflammation at feeding sites, and contributing to maceration, which decreases the barrier function of skin. Increased temperature and humidity have been hypothesized to play a role as well. Zoospores are attracted by low concentrations of carbon dioxide and repelled by high concentrations.37
Host factors that influence susceptibility to infection include poor body condition, malnutrition,38–40 stressful conditions, and glucocorticoids.41 Resistance in some animals may have a genetic component and appears to be associated with major histocompatibility complex (MHC) haplotypes and variation in serine composition.37,42
Immune Responses to Dermatophilus
Both humoral and cell-mediated responses are triggered by infection with D. congolensis.43–47 Resistance to infection may be related to antibody production,48 T-cell activation,49 and nonspecific immune mechanisms, such as epidermal hyperproliferation and neutrophil chemotaxis.50–52 Several reports, however, indicate that no correlation exists between serum antibodies and resistance53,54 Therefore, it is likely that location of antibodies may be more important than serum titers.
As the hyphae of D. congolensis spread in the epidermis, antigens are released that are captured by Langerhans’ cells and presented to T cells at the site of infection. Dense accumulations of mononuclear cells are found in infected skin. Chronic lesions contain both CD4+ and CD8+ T lymphocytes in equal proportions, whereas acute lesions on their way to resolution contain primarily CD4+ T cells.55 Cytokines secreted by T cells may be responsible for epidermal proliferation.
CLINICAL FINDINGS
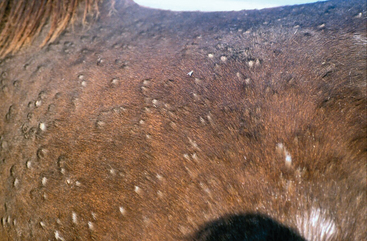
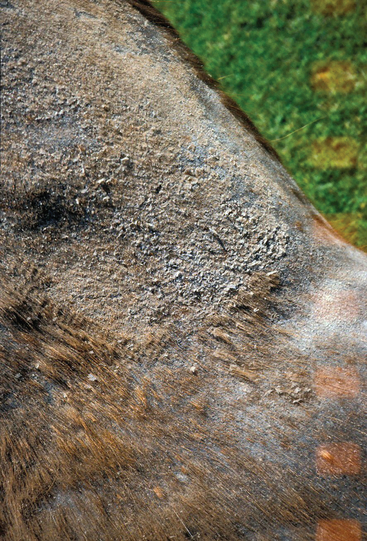
The clinical features of dermatophilosis are fairly characteristic.56 The primary lesions are papules, which mature into pustules. Because pustules are transient, only epidermal collarettes and areas of focal alopecia are often seen (Fig. 31-1). Lesions easily become exudative, and hairs are matted together to form thick crusts in which the hairs are embedded57 (Fig. 31-2). When crusts are removed, the underlying skin is often eroded, painful, and prone to bleeding (Fig. 31-3). Purulent exudate may be observed in active lesions, whereas chronic lesions tend to have dry crusts with more diffuse scaling and alopecia (Figs. 31-4 and 31-5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree