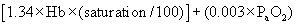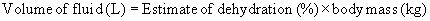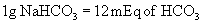CHAPTER 7 Critical Care
EQUINE INTENSIVE CARE UNIT
The decision to offer ICU services should be based on the population needs and the economic environment of the hospital and community. Several recent studies describe the general case population and commonly performed procedures and treatments of patients admitted on an emergency basis to large university referral centers.1,2 Because equine emergencies are relatively common among patients requiring critical care, such information provides an initial database for understanding population dynamics. In general, these studies revealed that although acute abdominal crisis is the most common type of case encountered, many cases will not require surgical intervention. Also, a variety of other problems presented as emergencies, and the required skills to deal with these included experience with dysfunction of most all body systems. Reviewing the anticipated distribution of cases within a given area, as well as the monthly distribution of cases, is important. Such reviews ensure an appropriate allocation of staff and equipment tailored to the needs of the population.
The equipment used for the care of critically ill equine patients should be based on the anticipated case population and the maximal level of care required for that population. Purchasing a ventilator would be a poor economic decision if mechanical ventilation is performed rarely. Renting medical equipment that might be needed only occasionally or seasonally may be a more practical choice. Box 7-1 lists some equipment to consider when equipping an equine ICU. Regular review of equipment and training for all personnel on new equipment are essential.
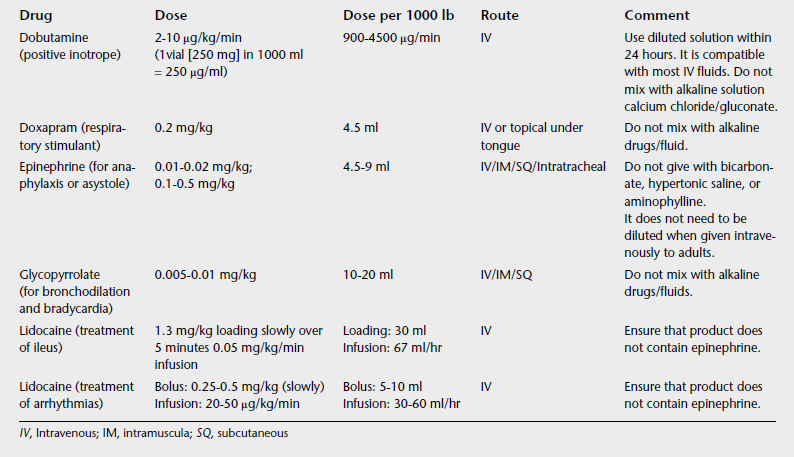
Emergency drugs should be readily available and mobile, possibly in a crash cart or box in the ICU. Table 7-1 lists several emergency drugs and dosages used in adult horses. Keeping a specific list such as this one readily available in the crash cart for easy reference is advisable. In addition, assembling packs for specific anticipated emergency situations, such as all the necessary items for delivering supplemental oxygen or supplies to perform an urgent tracheotomy, and placing these packs in key areas are recommended.
Oxygen ports for supplementation through nasal insufflation or for mechanical ventilation should be available. When installing a new ICU, the practitioner should consider placements of ports for oxygen, vacuum, and air, as well as a remote gas source and a pipeline system to allow delivery of oxygen. Compressed gas cylinders can be used, but these must be stored and handled appropriately to prevent injury. For each cylinder type, knowledge of the capacity of the cylinder and the flow rate enables calculation of the amount of time provided. The small portable E cylinders contain 655 L of oxygen when full and can provide oxygen for 260 minutes when set at a flow rate of 5 L/min. Adult horses may require flow rates of 10 to 15 L/min to have any significant effect on the fraction of oxygen inspired (FIO2). Larger G or H cylinders containing 5290 or 6910 L, respectively, allow oxygen supplementation for longer periods.
Although grouping critically ill patients in a single location allows for the greatest efficiency of personnel and equipment, maintaining biosecurity is essential. Grouping patients with similar disorders is therefore recommended. Strict disinfection and isolation protocols should be in place to prevent nosocomial infections and the spread of infectious disease. Thorough hand washing is an important component of most biosecurity protocols. Hand rubbing with an alcohol-based solution is more effective than hand washing with an antiseptic, probably because it does not require rinsing and drying of hands.3 Adoption of this practice significantly reduces cross-contamination among patients.
COMMON PHYSIOLOGIC FEATURES OF CRITICAL ILLNESS
Despite differing problems, the common denominator in the treatment of many critically ill patients is the need to maintain adequate delivery of oxygen to meet metabolic demand. Methods to return to a fully functional state include serial systemic evaluations, even when the problem appears focal; attention to minimize adverse affects of treatments; and restoration of homeostasis, in particular working toward adequate delivery of oxygen and nutrients to cells.
A more severe consequence of focal problems causing systemic effects can occur when the initial insult is recognized as foreign by the body, and the various components of the immune system become activated to eliminate the threat. Most of the time this process is appropriate, and the various inflammatory mediator or coagulation cascades are activated in an orderly fashion. However, sometimes the progression is not balanced, and activation of certain systems, such as those governing inflammation and coagulation, can lead to unchecked release of mediators and a generalized reaction. In sick horses the most commonly cited example of this is systemic inflammatory response syndrome (SIRS), which can occur as a consequence of endotoxemia. These aspects of equine medicine are discussed in detail in Chapter 15.
BASIC STEPS
An understanding of the physiologic and pathologic processes that may be occurring in critically ill patients is key to a successful outcome. The goal is to evaluate the patient, recognize current problems, anticipate impending issues, and then create an action plan. Because critical illness is generally dynamic, the plan also must also be dynamic. In the often highly emotionally charged period of initial patient evaluation, a systematic, thorough approach to each patient is beneficial. A basic 12-step plan for assessing and treating a critically ill patient is outlined in Box 7-2.
BOX 7-2 TWELVE-STEP PLAN FOR CRITICAL CARE ASSESSMENT AND TREATMENT
VASCULAR ACCESS AND ADMINISTRATION OF FLUIDS
Intravenous catheters are available in varying materials, constructions, lengths, and diameters (Table 7-2). In choosing a catheter, the practitioner should consider the desired fluid rate, fluid viscosity, the length of time the catheter will remain in the vein, the severity of the systemic illness, and the size of the animal. The rate of fluid flow is proportional to the diameter of the catheter and inversely proportional to the length of the catheter and the viscosity of the fluid. Standard adult horse catheter sizes are usually 14 gauge in diameter and 5.25 inches in length. For more rapid administration rates, larger-bore catheters, such as 12- or 10-gauge, could be used, with the caveat that these larger sizes may be more traumatic to the vascular endothelium, which increases the risk of thomobosis, Plasma, blood products, and synthetic colloids, because of their increased viscosity, flow more slowly; if the horse requires volume replacement, the practitioner can combine administration of these fluids with a balanced electrolyte solution.
TABLE 7-2 List of Available Catheter Materials
| Material | Example | Comment |
|---|---|---|
| Polypropylene | PE tubing, Medicut | Highly thrombogenic not recommended |
| Teflon | Angiocath | Less thrombogenic |
| Polyurethane | Mila | Much less thrombogenic |
| Silastic | Centrasil | Least thrombogenic |
One also must consider the catheter construction (Table 7-3). Through-the-needle catheters are most common for standard-size adult horses. An over-the-wire catheter is best used in foals and miniature horses or when catheterizing the lateral thoracic vein. Short and long extension sets are available, as well as small- and large-bore diameters. Using an extension set that screws into the hub of the catheter is best, to prevent dislodgment. In horses with low central venous pressures (CVPs), disconnection of the line can result in significant aspiration of air and cardiovascular collapse. Double extensions are available for horses that require administration of other medication with the fluids.
In adult horses a catheter is usually not covered with a bandage so that potential problems can be quickly identified. The practitioner may need to apply bandages to foals if they are tampering with the catheter. A triple-antibiotic ointment may be applied at the insertion site to decrease infection. Catheters should be flushed with heparinized saline (10 IU/ml) four times per day if they are not used for fluid administration. When administering a medication, the clinician should wipe the injection cap with alcohol before inserting the needle and change the injection cap daily. The clinician should culture catheters if catheter site infection is suspected for identification of the causative organism and to facilitate early recognition of possible nosocomial infection.
BASIC FLUID THERAPY
Designing a Fluid Therapy Regimen
DEHYDRATION
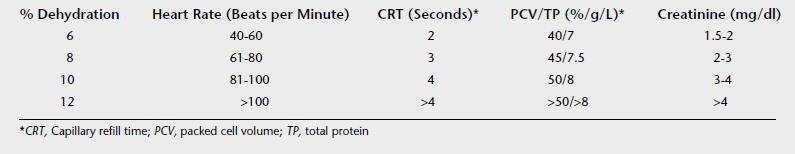
Evaluation of dehydration is at best a subjective estimate, and the clinician must understand that this estimate must be adjusted on the basis of monitoring parameters. Table 7-4 lists useful parameters for evaluating acute, extracellular dehydration.
ONGOING LOSSES
Sometimes the clinician can measure and record ongoing losses (e.g., for nasogastric reflux), but ongoing losses usually must be estimated. The clinician monitors the patient to determine whether the calculated fluid volume is meeting the ongoing losses. Patients receiving fluids intravenously should be monitored at least twice a day, including assessment of heart rate and measurement of packed cell volume and total protein; the clinician should monitor patients more frequently (every 2, 4, or 6 hours) depending on the severity of cardiovascular compromise. The clinician should also monitor creatinine concentration once daily when initially elevated to ensure adequate return to normal. Additional means of monitoring adequate fluid delivery may include measurement of CVP, arterial blood pressure, and urine output.
TYPE OF FLUID
Two categories of fluids commonly are used for fluid replacement: 0.09% saline and balanced electrolyte solutions (BESs). Table 7-5 lists the composition of various commercially available fluids. In general, BESs are chosen when serum electrolytes are close to normal. All BESs contain some potassium. Saline is higher in sodium and much higher in chloride than serum concentrations and is used when sodium is lower than 125 mEq/L. Saline also is used in disease processes associated with high potassium levels, such as hyperkalemic periodic paralysis or renal failure, in which a potassium-free solution is preferable. In cases of long-term fluid maintenance therapy (greater than 4 to 5 days), if the oral route is not available, the practitioner should consider half-strength basic fluids to which potassium and calcium are added. Long-term fluid therapy with routine BESs results in hypernatremia, hypokalemia, hypomagnesemia, and hypocalcemia.
In horses routine fluid replacement includes calcium and potassium supplementation, in particular when the horse receives no oral intake because of gastrointestinal disease. Both electrolytes are important for smooth muscle function and vascular tone. Recently, magnesium supplementation also has received interest, particularly with fasting and ileus.1,2
In chronic metabolic acidosis, particularly with ongoing losses of bicarbonate (e.g., diarrhea), the horse usually requires the full calculated amount, partly because the bicarbonate loss is distributed over all fluid compartments, not just the extracellular fluid. Orally administered bicarbonate is a good means of dealing with ongoing losses in horses with diarrhea.
Bicarbonate can be given orally as a powder, where
ORALLY ADMINISTERED FLUIDS
Oral fluid therapy should be administered using the fluid composition shown in Table 7-6. One should administer calcium separately because it causes precipitation of the solution. This electrolyte solution meets daily needs for an adult horse and can be given through a small, preplaced nasogastric feeding tube or by intermittent intubation.
TABLE 7-6 Fluid Composition for Orally Administered Fluid Therapy
| For Every L of Water, add: | |
| FOR EVERY 21 L OF WATER | |
| Electrolyte | Amount |
| NaCl | 10 g |
| NaHCO3 | 15 g |
| KCl | 75 g |
| K2HPO4 | 60 g |
Fluids Used to Expand Circulating Blood Volume
COLLOIDS
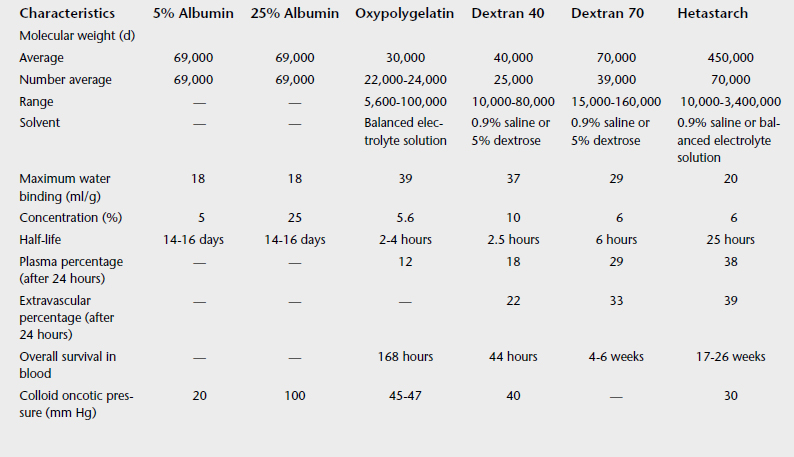
Colloids are fluids that contain a molecule that can exert oncotic pressure. These molecules do redistribute to the extracellular fluid but at a much slower rate than crystalloids, so that the duration of effect is prolonged compared with that of crystalloids. Table 7-7 describes the different colloids available.
A disadvantage of natural colloids (plasma or albumin) is that they are more antigenic and can cause allergic reactions. Synthetic colloids have a much lower antigenicity, but they can cause bleeding disorders because of their tendency to coat platelets or by causing a decrease in coagulation factor. In the horse Dextran 40 can cause anaphylactoid reactions. Hetastarch administration can cause a decrease in coagulation factors and prolong clotting times, particularly at high doses (20 ml/kg).3 The dose is 10 ml/kg of 6% solutions.
BLOOD SUBSTITUTES
Blood substitutes are hemoglobin solutions. Currently, only one commercial hemoglobin solution (oxyglobin, which is made from bovine hemoglobin) is available. The major advantage of oxyglobin is that it does not depend on 2,3-diphosphoglycerate for oxygen-carrying capacity, such that it can be stored and is immediately able to transport oxygen. The duration of effect is approximately 18 hours in horses, after which point another dose or a blood transfusion must be considered.4,5 Unfortunately, cost limits the usefulness of oxyglobin in horses.
WHOLE BLOOD
Ideally, the ICU should maintain blood donors that are free of antigenic determinants, particularly Aa and Qa, and of isoantibodies. The practitioner can perform a major and minor crossmatch to select an appropriate donor, provided that complement is added to the test for hemolysin detection. Interpretation of the minor crossmatch may be difficult if autoagglutination is present. If crossmatch is unavailable, one can use a non-Thoroughbred gelding. A volume of 20 ml/kg can be safely collected every 3 weeks in adult horses,3 and whole blood can be collected in sodium citrate using sterile technique. Commercial blood collection kits are also available. Complications of blood transfusion include acute anaphylactic reactions, allergic reactions, hemolysis, fever, tachypnea, and hypocalcemia caused by citrate chelation.
MONITORING ARTERIAL BLOOD PRESSURE
Direct or Invasive Blood Pressure Measurement
Suitable arteries for arterial catheterization in the horse include the transverse facial, facial, and greater metatarsal arteries. In the standing horse the transverse facial or the facial artery are most practical (Figure 7-1).
Indirect Measurement
OSCILLOMETRIC SPHYGMOMANOMETRY
Oscillometric sphygmomanometry relies on the recording of the change in oscillations generated by the change in blood flow during deflation of the cuff. Oscillations start as the cuff pressure reaches systolic pressure, are maximal at mean pressure, and disappear when the cuff reaches diastolic pressure. The meter records and displays systolic, diastolic, and mean pressures. When placing the meter on the tail, one can use tape or a bandage below the cuff to prevent its slipping, but should not restrain the cuff itself (Figure 7-2).
TRANSESOPHAGEAL ULTRASOUND
Transesophageal ultrasound is a method of hemodynamic monitoring that uses a transesophageal probe with two ultrasound transducers (HemoSonic; Arrow International). An M-mode transducer continuously measures the aortic diameter, and a pulsed Doppler measures the flow velocity, providing hemodynamic measurements, including MAP. Although transesophageal Doppler echocardiography has been evaluated for determination of cardiac output in horses, the accuracy and repeatability of the combined probes for blood pressure determination have not been evaluated (Vingmed Sound, Horten, Norway).
TRACHEOSTOMY
Box 7-3 lists the materials needed for an emergency tracheostomy pack. If possible, the clinician should clip, prepare, and infiltrate with local anesthetic the planned incision site. In cases of acute respiratory distress, this may not be possible, however. The clinician makes an 8- to 10-cm longitudinal incision at the junction of the proximal and middle third of the neck, just above the V made by the junction of the sternothyrohyoideus muscles. It is important to stay on midline to favor drainage, separating the sternothyrohyoideus muscles on the midline and exposing the trachea. The clinician makes a transverse incision between two tracheal rings, taking care not to damage the tracheal cartilages. If the head of the horse is supported in elevation during the procedure, the clinician should make the tracheal incision distal in relationship to the skin incision to keep from covering the incision when the head is lowered. In emergency situations a J-type tracheostomy tube is used because of its ease of insertion. When the horse is calm, or if the situation is not critical, a self-retaining tube is preferable for maintenance because J-tubes tend to fall out. If the animal is to be ventilated, a silicone-cuffed tube is preferable to allow for closed-system ventilation.
THORACOCENTESIS
In open pneumothorax sealing of the chest must occur, followed by evacuation of air. The clinician can seal the chest with sheets of plastic wrapped around the site of entry or close the wound if possible. The chest is evacuated by placing a small trocar, or a 14-gauge catheter, in the dorsal twelfth intercostal space, and the trocar is removed once the air has been evacuated satisfactorily. In closed pneumothorax or tension pneumothorax, the catheter must be kept in place until the source of air entry can be sealed.
NASOGASTRIC INTUBATION
The clinician should note the amount of reflux obtained because this factors into ongoing losses, and the volume of fluids given intravenously must be adjusted accordingly. Horses with functional ileus need gastric decompression usually every 4 hours, although if the condition is severe, they may require decompression every 2 hours. The nasogastric tube should be left in place only as long as required because some horses develop pharyngeal and laryngeal irritation associated with its presence.6 These horses then have pain when swallowing when they resume feeding.
ABDOMINOCENTESIS
Abdominocentesis is important for evaluating abdominal disease, whether it is colic, weight loss, or postoperative problems. Box 7-4 provides the materials needed to perform this procedure.
Enterocentesis sometimes occurs and must be differentiated from intestinal rupture. With enterocentesis a cytologic examination reveals plant material, bacteria, and debris but no cells. The clinical condition of the horse is not consistent with rupture, although in early rupture clinical signs may not reflect rupture (2 to 4 hours are necessary for manifestation of signs). Cytologic examination of abdominal fluid with intestinal rupture shows neutrophils, bacteria, and bacteria that have been phagocytized by neutrophils.
TROCARIZATION OF THE LARGE COLON
Trocarization of the large colon is occasionally useful to decompress the abdomen for abdominal compartment syndrome (i.e., severe distention associated with pain and dyspnea). Box 7-5 lists the materials needed for this procedure.
REVIEW OF CARDIOVASCULAR PHYSIOLOGY
Cardiac output, the volume of blood pumped through the heart per minute, is calculated as the product of heart rate and stroke volume. Heart rate is determined by a number of neurologic, endocrinologic, and physical factors. Stroke volume is the amount of blood expelled from the heart with each contraction (i.e., the difference between end-diastolic volume [maximal filling] and end-systolic volume within the ventricle [peak contraction]). The quantity of blood arriving as the arterial supply of a tissue depends on cardiac output.
CARDIOVASCULAR INSUFFICIENCY: SHOCK STATES
If the cardiovascular system fails to meet the demands of the animal for adequate oxygen delivery to tissues, a state of shock ensues. Shock is classified into four major types: hypovolemic shock, cardiogenic shock, obstructive shock, and distributive shock.1 The first three of these categories of shock are associated with a decrease in cardiac output and uniform circulatory disturbances in the arterioles, venules, and capillaries. Anaerobic tissue metabolism ensues as a result of diminished oxygen delivery. The fourth type of shock, distributive or vasodilatory shock, is associated with a heterogenous disturbance of blood flow in the microcirculation and areas of shunting.2
Cardiogenic shock is the result of impaired ventricular function with resultant decreased cardiac output. Myocarditis, myocardial infarction, valvular pathology, or arrhythmias may be responsible.3 Inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin (IL)-2 and IL-6, decrease the contractility of cardiac myocytes, effects shown experimentally to be mediated by nitric oxide.4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree