Fig. 1.
Preparation of the cranial window—exposure of both hemispheres.
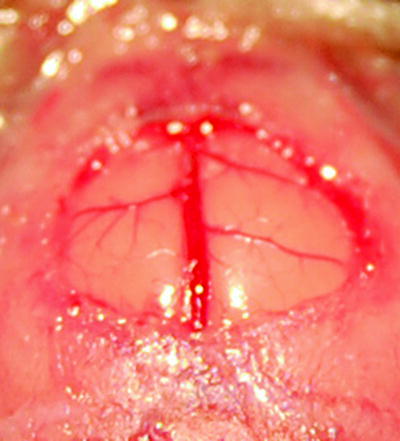
Fig. 2.
Enlarged view of the window preparation showing the superior sagittal sinus and the bridging veins of both hemispheres.
1.
The animal is anaesthetized with an intraperitoneal injection of ketamine (1.2 mg/10 g body weight) and xylazine (0.16 mg/10 g body weight).
2.
The anaesthetized animal is fixed in the stereotaxic head holder in a prone position.
3.
After hair removal and disinfection a mid-sagittal incision is made, exposing the frontal and parietal skull.
4.
Preparation of the galea and periost to each side until the onset of the temporal muscle is exposed.
5.
Cleaning with gauze.
6.
Drilling of a circular bone flap (diameter approx. 6 mm), overlapping the superior sagittal sinus under continuous irrigation and cleaning to avoid damage to the brain by heat or direct injury and to provide clear vision.
7.
Special attention should be paid to the drilling over the sagittal suture, where the sinus is adherent to the bone.
8.
Removal of the bone flap should be performed under highest magnification to avoid damage to the sinus or bridging veins.
9.
After removal of the bone flap cleaning is facilitated by irrigation and careful use of gauze.
10.
For dural opening and removal an intracutaneous needle can be used. The opening should be achieved at the far lateral region of the craniectomy.
11.
Bleedings should be stopped by irrigation only.
12.
Be aware that the brain surface is to be kept moist at all times.
13.
The window glass coverslip is threaded into position and fixed with glue. No air should be trapped beneath the glass slide.
14.
Skin closure is achieved by primary suture at the end of the procedure.
15.
Note that for assessment of inflammatory changes the first measurement should be performed at least 2 days after implantation of the window to eliminate the influence of the operative trauma.
3.2 Fluorescence Microscopy
Fluorescence microscopy is a widely used standard observation and evaluation technique for a variety of “windows” to the vasculature. It uses light of a certain wavelength to illuminate specifically applied fluorophores (e.g. green fluorescent protein, fluorescein, etc.). A filter filters the excitation light from the fluorescence light, thus giving a 2D fluorescent image with an improved signal-to-noise ratio.
Intravital fluorescence microscopy allows for analysis of the following parameters:
1.
Vascular length (L) and diameter (Dv).
2.
Luminal surface area (A = Dv × L × π).
3.
Mean blood flow velocity (V blood = V max/(2-(L/Dv)²), where V max represents the maximum leukocyte velocity.
4.
Wall shear rate (γ = V blood × 8/Dv).
5.
Wall shear stress (τ = γ × 0.025) 0.025 is assumed to be the blood viscosity under normal conditions.
6.
Total cellular flux (TF = number of cells passing a distinct vessel per observation period).
7.
Rolling flux (RF = number of rolling cells per observation period).
8.
Sticking flux (SF = number of stationary cells >20 s).
9.
Rolling fraction (RFx = RF/TF).
10.
Sticking fraction (SFx = SF/RFx), number of rolling cells that become firmly adherent.
11.




Locomotion of adherent cells on the endothelium.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


